Preparation magnesium diboride by magniathermal boron oxide based on superconducting properties
Автор: Alipbayev A.N., Suleimenova M.Sh., Boloskhan S.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Естественные науки
Статья в выпуске: 1 (126), 2020 года.
Бесплатный доступ
Results of receiving a diborid of magnesium are given in work by magniathermal oxidation of boron composite compounds at different argon pressures and temperatures. The relevance of a research is proved by superconducting properties of magnesium diboride. Synthesis of magnesium diboride itself accelerates, due to wall burning and thermal explosion of exothermic mixture of the reaction products of magniathermal. It is shown that the use of SHS method at high argon pressure and temperature allows to obtain magnesium diboride of high purity.
Magnesium diboride, self-propagating high-temperature synthesis (shs), boron oxide, thermal explosion, high pressure
Короткий адрес: https://sciup.org/140250863
IDR: 140250863 | УДК: 66.72
Текст научной статьи Preparation magnesium diboride by magniathermal boron oxide based on superconducting properties
БОР ОКСИДІНІҢ МАГНИЙТЕРМИЯ ӘДІСІМЕН АСА ӨТКІЗГІШ ҚАСИЕТТЕРІ БАР МАГНИЙ ДИБОРИДІН АЛУ
А.Н. АЛИПБАЕВ1, М.Ш. СУЛЕЙМЕНОВА1, С. БОЛОСХААН1
(1Алматы технологиялық университеті, Алматы қ., Қазақстан)
Бұл жұмыста магний диборидін аргонның әртүрлі қысымдарында және температурасында бордың композиттік қосылыстарының магнийтермиялық тотығуымен алу нәтижелері келтірілген. Зерттеудің өзектілігі магний диборидінің аса өткізгіш қасиеттерімен негізделген. Магний диборидінің синтезі қабырғалық жану және магнийтермия реакциясы өнімдерінің экзотермиялық қоспасының жылу жарылысы есебінен өздігінен жойылады. ӨЖС әдісін аргонның жоғары қысымы мен температураның кезінде пайдалану жоғары таза магний диборидін алуға мүмкіндік беретіні көрсетілген.
Негізгі сөздер: магний дибориді, ӨЖС- өздігінен тұтанатын жоғары температуралық синтез, бор оксиді, жылулық жарылыс, жоғары қысым.
Intrduction
Boron is widely used as fuel in jet engines and solid fuel engines due to its specific combustion characteristics [1-8]. Superconductivity of magnesium diboride was discovered recently in 2001 by Japanese scientists. A number of magnesium diboride synthesis pathways are known nowadays. For instance, synthesis under high pressure or thermal explosion synthesis, ultra-high-pressure synthesis, synthesis via mechanical activation, etc. Metal borides are inorganic substances with basic characteristics, exists in various states of matter, has metallic appearance. This substance can be identified by its exceptional electron structure, thermal conductivity, magnetic properties and ability to melt without decomposing due to high thermal stability. Recycling of magnesium diborideis restrained due to lack of research in synthesis and characterization of former compound. Synthesis of magnesium diborideis carried out in solid polycrystalline phase. One of the perspective methods is oxidation of boron anhydride by magnesium-thermic method. Elemental magnesium is quite reactive and volatile substance with low boiling point, therefore oxidation I carried out in sealed reactor under the high pressure of inert atmosphere. On the other hand, self-propagating high-temperature synthesis (SHS) and mechanochemical activation methods are drawing attention in recent years.
Оbject and methods of research
The experiments were conducted in an experimental setup providing pressurization up to 10 MPa with inert gas. Synthesis temperatures were determined by thermocouples with recording of the data in PC. To increase the concentration limits of SHS-synthesis, a tube heating furnace was placed inside the reactor, thus allowing pre-heating the sample under study up to 10000C. SHS products were characterized by the method of X-ray phase analysis.
Results and analysis
The literary review showed that magnesium diboride can be received magniathermal oxidation of boron-containing connections.
The experiment shows that magnesium diboridecan be synthesized from boron oxide (III):
4Mg + B 2 O 3 = MgB 2 + 3MgO (1)
As shown in thermodynamic calculations reaction (1) results in formation of magnesium diboride. Based on calculations, standard Gibbs energy is equal to - 562.9 kJ/mole, i.e. reaction (1) is exothermic.
ΔG(1) = (ΔG(MgB 2 ) + 3ΔG(MgO) – (4ΔG(Mg) + ΔG(B 2 O 3 )) = - 562.9 kJ/mole
Magnesium and boron oxide (III) were mixed in stoichiometric ratio and grinded in АРГО-2 planetary ball mill. After that, in accordance with ТЕ method, cylindrical samples with 1.5 cm diameter and 2 cm height were prepared from powder. These samples were heated in reactor at 750-800°C, and under 20-35 atm pressure. During heating, when reached to 750-
800°C, samples underwent either heat explosion or SHS synthesis. Figure 1 shows gradual lowering of argon atmosphere pressure when combustion temperature is between 950-1200°C.
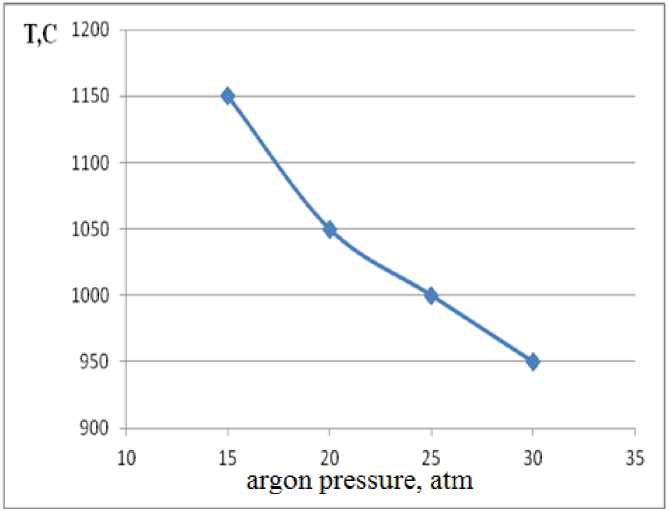
Figure 1 - Dependence of combustion temperature and argon pressure in Mg-B 2 O 3 system
Mekting point of magnesium lies in this range, more precisely at 1007°C. Intense melting at this temperature increases temperature of burning and a zone of reaction of magnesium and leads to increased yield of magnesium diboride.
Dependence between combustion temperature and argon pressure is shown in figure 1. By extrapolating this curve, theroetical values for combustion temperature and argon pressure can be estimated. For instance, at pressure levels 10 atm, combustion temperature is 1200°C.
According to thermodynamic equations, Gibbs free energy change in reaction (1) is -562.9 kJ/mole, that is, reaction is exothermic, however in practice reaction is not spontaneous. Therefore, highly reactive compounds can be used to initiate the reaction. These include potassium chlorate.
Following are main reactions of magnesium diboride synthesis using potassium chlorate:
3Mg + KClO 3 = 3MgO + KCl + Q
B 2 O 3 + 3Mg = 2B + 3MgO
Mg + 2B = MgB 2
4Mg + B 2 O 3 = MgB 2 + 3MgO
Net reaction: KClO 3 = KCl + O 2
Thermodynamic initiation steps:
1)O 2 = O- + O-
2)O^ + MgB2 = MgO +B- з)в^ + MgO = MgB2 + O^
4)O- + O^ = O 2
2L7y- = 2k 1 [O 2 ] - k 2 [O^][MgB 2 ] + k 3 [B^][MgO] - k 4 [O^2
= k 2 [O^][MgB 2 ] - k 3 [B-][MgO] + ^ = 0; 2k 1 [O 2 ]= k 4 [O^]2
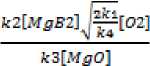
[O^] = . W-J [B^] =
^-^ = k 1 [O^][ MgB 2 ] - k 3 [B^][ MgO] = 0; k 1 [O^][ MgB 2 ] = k 3 [B^][ MgO]
kirol kl W02lj 1 kl
[MgB2] = кЗ [В •] [MgO] ; [MgB2] = [wS ; [MgB2] = И[МЯВ2]
[MgB 2 ]/dt=W =
X-ray phase analysis results show content of magnesium diboride in final product to be as high as 25% when synthesized in the presence of KClO 3 .
Table 1 - X-ray phase analysis results for combustion products of Mg-B 2 O 3 system
|
Mg and B 2 O 3 mixture mechanical activation time |
Mass percentage of components |
Products of synthesis, % |
|||||||
|
Mg |
B 2 O 3 |
KClO 3 |
MgO |
Mg |
MgB 2 |
KClO 3 |
KCl |
Mg 3 (BO 3 ) 2 |
|
|
30 |
47 |
44 |
9 |
57.5 |
7.8 |
22.5 |
1.9 |
3.2 |
7.1 |
|
90 |
49 |
42 |
9 |
55.9 |
7.5 |
24.7 |
1.1 |
4 |
6.8 |
The composition of synthesis products implies efficiency of synthesizing magnesium diboride via magnesium-thermal process in presence of potassium chlorate. X-ray phase analysis results show presence of magnesium diboride as well as magnesium borate. Along with that 25% magnesium diboride content in the product is reached when mass ratio of Mg:B 2 O 3 :KClO 3 is 47:44:9, while theoretical yield is equal to 91%.
The synthesis products did not provide the optimal success of the magnesium diboride mass fractionation by mixing a small amount of acid for the removal of the oxygen layer from the magnesium oxide on the phase.
Superconductive properties can be obtained by dissolving magnesium oxide in the final product and lowering the content of magnesium borate in the product via forming amorphous substance from latter.
In addition, it was shown that the density of the currents (J c ) was calculated by the formula of the viney, while the second account was characterized by the conductivity of Mg: B 2 O 3 : KClO 3 .
Table 2 - Comparison of superconductivity parameters for different systems
|
Names |
Critical temperature Т с , К |
Critical current density J c , A/см2 |
||
|
Without an internal pole |
From the 2T effect of the inner pole |
From the 4T effect of the inner pole |
||
|
Mg + B system |
39.07 |
2∙108 |
1.2∙106 |
0.8∙104 |
|
Mg + B 2 O 3 system |
36.01 |
0.9∙105 |
0.3∙102 |
0.08∙102 |
Superconductivity of product occurs at 36.01 K as a result of critical temperature, while current density under magnetic field is equal to 0.08∙102 A/cm2. As shown in table 2, Mg + B system has more prominent superconductive properties. The aim of this study is synthesizing magnesium diboride via SHS. Content of products were verified using X-ray phase analysis. Superconductive properties magnesium diboride synthesized by two different methods were compared.
Conclusion
On result of the X-ray phase analysis it is proved that purity of magnesium diboridevery high.At the same time, magnesium thermal method of synthesizing magnesium boride from magnesium and boron oxide via mechanical activation resulted in high yield of magnesium diboride. As rate of magnesium diboride formation strongly depends on primary or secondary reaction, reaction was rapid and formation of magnesium oxide layer on the surface of magnesium was proven by X-ray phase analysis. Composite materials from boron-containing components are received by the SНS method with high pressures of argon.Obtained products exhibit superconductive properties.
Список литературы Preparation magnesium diboride by magniathermal boron oxide based on superconducting properties
- A. N. Alipbaev, R. G. Abdulkarimova, S. M. Fomenko, Z. A. Mansurov, V. E. Zarko.Getting magnesium diboride by SHS under high pressure of argon//NAS RK chemistry and technology, Volume 2. - Number 416. 2016.- PP. 11-16 [In Russian].
- Nagamtsu J., Nakagawa N., Muranaka T. J. Superconductivity at 39 K in magnesium dibo-ride//Nature journal, V. 410, N 6824, 2001. -PP. 63-64.
- Ivanovsky A.L. Superconducting MgB2 and related compounds: synthesis, properties, electronic structure //Advances in Chemistry, 2001. - V.70. - P.811-829 [In Russian].
- Korchagin A.M., Zarko V.E., Fomenko S.M., Alipbaev A.N., The effect of preliminary mechanical processing of Mg and B mixture on the parameters of its thermal explosion and reaction products characteristics// Proceedings of the isreal annual conference on aerospace sciences, tel-aviv& Haifa, isreal march 9-10,2016. - PP. 65-72.
- Zarko V.E., Fomenko S.M., Alipbaev A.N., Abdulkarimova R.G. Synthesis of nanopowders of magnesium diboride by magnesium thermic reduction under the conditions of high pressure of argon// Key engineering materials, 2016. - №2. -РР. 56-63.
- Korchagin A.M., Zarko V.E., Fomenko S.M., Alipbaev A.N., Mansurov Z.A. Laboratory production of MgB2 by thermal explosion of mechnoactivated Mg-B mixes// Nature V.210. - PP. 3517-3522.
- Perminov V.P., Neronov V.A., Mali V.I. Explosive synthesis of compounds in boron systems silicon and boron magnesium // Metallurgy, 2006. - V.4. - PP. 31-45 [In Russian].
- Rogachev A.S., Kochetov N.A., Kurbatkina V.V. Microstructural aspects of gasless combustion of mechanically activated mixtures. High-speed microvideo survey of Ni + Al composition //Combustion and explosion physics, 2006. - V.4. - PP. 62-71 [In Russian].


