Prevalence of left ventricular diastolic dysfunction by tissue Doppler imaging in patients with end stage renal disease undergoing regular dialysis with preserved systolic function
Автор: Sallam W.M., Elkaialy A.A., Gharieb M.A., Thabet S.S.
Журнал: Cardiometry @cardiometry
Статья в выпуске: 33, 2024 года.
Бесплатный доступ
Background: Patients with end-stage renal disease (ESRD) undergoing regular dialysis face a heightened risk of cardiovascular complications, with left ventricular (LV) diastolic dysfunction being a significant concern. Despite preserved systolic function, diastolic dysfunction can lead to notable morbidity. Tissue Doppler imaging (TDI) is a reliable noninvasive tool for assessing diastolic function. This study aims to investigate the prevalence and severity of LV diastolic dysfunction in ESRD patients undergoing regular dialysis with preserved systolic function.
Esrd, diastolic dysfunction, tissue doppler imaging, hemodialysis, e/e’ ratio
Короткий адрес: https://sciup.org/148330039
IDR: 148330039 | DOI: 10.18137/cardiometry.2024.33.8794
Текст научной статьи Prevalence of left ventricular diastolic dysfunction by tissue Doppler imaging in patients with end stage renal disease undergoing regular dialysis with preserved systolic function
Walid Mohamed Sallam, Ahmed A. Elkaialy, Magdy A. Gharieb, Sameh S. Thabet. Prevalence of left ventricular diastolic dysfunction by tissue doppler imaging in patients with end stage renal disease undergoing regular dialysis with preserved systolic function. Cardiometry; Issue No. 33; November 2024; p. 87-94; DOI: 10.18137/cardiometry.2024.33.8794; Available from: prevalence-left-ventricular0diastolic
Chronic Kidney Disease (CKD) has become a significant global public health issue due to its increasing incidence and prevalence 1. As CKD progresses to end-stage renal disease (ESRD), the risk of cardiovascular events and complications rises sharply 2. Cardiovascular mortality in patients with ESRD undergoing renal replacement therapy is notably higher, with rates that are 10 to 20 times greater than those observed in the general population 3. A key cardiovascular complication associated with CKD is the development of left ventricular (LV) hypertrophy and fibrosis, which contribute to ventricular stiffness and impaired relaxation. These changes can lead to increased pressures in the left atrium (LA) and pulmonary veins, often resulting in pulmonary edema, even when left ventricular systolic function is preserved 4.
Left ventricular hypertrophy (LVH) emerges early during the progression of CKD and is often accompanied by myocardial fibrosis. This condition represents an independent risk factor for mortality in CKD patients5, 6. Several factors contribute to myocardial damage, including both preload-related elements such as intravascular volume expansion, anemia, and arteriovenous fistulas in hemodialysis patients, and afterload-related factors like systemic arterial resistance and vascular calcification. These alterations lead to eccentric and concentric LV remodeling, contributing to the development of diastolic dysfunction, ultimately manifesting as heart failure with preserved ejection fraction (HFpEF) 7.
In addition to hemodynamic factors, non-hemodynamic contributors, such as anemia, hyperphosphatemia, secondary hyperparathyroidism, and vitamin D deficiency, further exacerbate myocardial fibrosis in patients with CKD 8. Secondary hyperparathyroidism, in particular, plays a critical role by impairing LV compliance, inducing myocardial calcification, and promoting LVH through increased arterial stiffness. As a result, diastolic dysfunction becomes established, creating a clinical scenario where noninvasive diagnostic approaches, such as Doppler echocardiography, are essential for assessing LV filling pressure 9, 10.
The goal of this study is to evaluate the impact of regular dialysis on LV diastolic function in ESRD patients, utilizing TDI to assess diastolic dysfunction in this population.
PATIENTS AND METHODS
This observational study was conducted from January 2020 to August 2020, involving 100 patients diagnosed with ESRD who were on regular hemodialysis over six months.
The study included patients diagnosed with ESRD according to the National Institute for Health and Care Excellence (NICE) clinical guidelines (2017) 11. These patients had a glomerular filtration rate (GFR) of less than 15 ml/min/1.73 m² and had been undergoing regular dialysis for more than six months. The institutional review board approved the study, and written informed consent was obtained from all patients.
Exclusion criteria
Patients were excluded from the study if they were 80 years or older, had unstable conditions such as cardiogenic shock, or had LV systolic dysfunction with an EF below 50%. Additional exclusion criteria included atrial flutter, atrial fibrillation, frequent premature ventricular contractions, and significant primary valvular disease (grade 3 or 4 mitral or aortic regurgitation, or severe mitral or aortic stenosis). Patients with regional wall motion abnormalities (RSWMAs) detected on a 2D echocardiogram, poor echocardiographic image quality, or indeterminate diastolic dysfunction were also excluded
Methods
Each participant underwent a thorough clinical evaluation, including a detailed medical history, physical examination, electrocardiogram (ECG), and
88 | Cardiometry | Issue 33. November 2024
blood sampling to measure creatinine and hemoglobin levels.
Echocardiographic evaluation was conducted using GE ultrasound systems to assess LVEF, LV dimensions, and LAVI. Measurements were obtained from the parasternal long-axis and apical four-chamber views using M-mode and 2D imaging techniques. Pulsed wave Doppler was utilized to analyze the E and A waves, the E/A ratio, and deceleration time. Continuous wave Doppler was used to measure tricuspid regurgitation velocity (TR vmax) across the tricuspid valve. Finally, TDI was employed to assess the e’ wave and calculate the E/e’ ratio, providing a comprehensive assessment of LV diastolic function. Figure 1
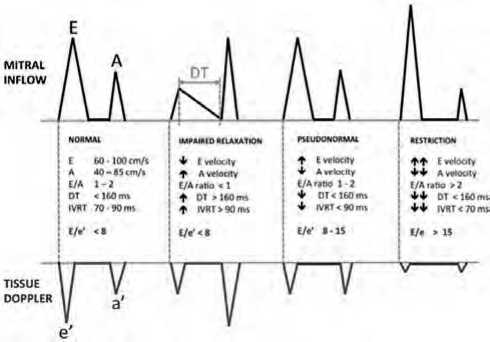
Figure 1: Assessment of diastolic function using PWD across the mitral valve and TDI along the mitral valve lateral annulus 12 .
The evaluation of LV diastolic function was categorized into four levels: normal diastolic filling, grade I diastolic dysfunction, grade II diastolic dysfunction, and grade III diastolic dysfunction, according to the data collected. Interpretation followed the guidelines set by the American Society of Echocardiography (ASE), as detailed by Nagueh et al. 12. n grade I diastolic dysfunction, also known as impaired relaxation, the mitral E/A ratio is less than 0.8, e’ velocity is below 8 cm/second, the E/e’ ratio is less than 8 (averaged from septal and lateral annulus), and the LAVI may be normal or slightly elevated. As the condition worsens, LV compliance decreases, and filling pressures increase, resulting in grade II diastolic dysfunction, or pseudonormalization. This stage is characterized by an E/A ratio between 0.8 and 2, an e’ velocity below 8 cm/second, an E/e’ ratio between 9 and 12, and a LAVI exceeding 34 ml/m².
In cases of advanced diastolic dysfunction, classified as grade III, a restrictive LV filling pattern is pres- ent. This is indicated by an E/A ratio greater than 2, an e’ velocity less than 8 cm/second, an average E/e’ ratio exceeding 13 (or septal E/e’ greater than 15), and a left atrial volume index (LAVI) surpassing 34 ml/m², often exceeding 40 ml/m². This stage reflects the highest LV filling pressures and is associated with the worst prognosis, particularly when the restrictive filling pattern persists and does not improve with appropriate therapeutic interventions.
b)
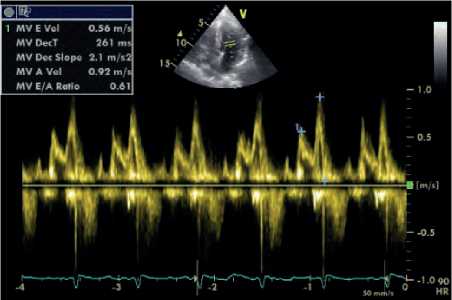
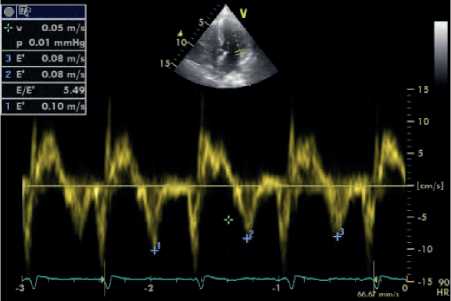
Figure 2: Assessment of diastolic function using PWD across the mitral valve (a) and TDI along the mitral valve lateral annulus (b) (From an ESRD patient).
Statistical analysis
Data management and statistical analysis were conducted using SPSS software, version 26 (IBM, Armonk, NY, USA). Continuous data were expressed as mean ± standard deviation, whereas categorical variables were presented as percentages. The chi-square test was used to analyze changes in categorical variables, with statistical significance determined at a P-value of <0.05. Quantitative data were described by their mean, standard deviation (SD), and range, whereas qualitative variables were represented as frequencies and percentages. Spearman’s correlation coefficient was used to evaluate the relationships between variables, classifying them as either positive or negative.
RESULTS
The study enrolled 100 ESRD patients, diagnosed according to the 2017 NICE clinical guidelines (with a GFR of 15 ml/min/1.73 m²), all of whom had been receiving regular dialysis for more than six months. Demographic and clinical characteristics of the studied patients were shown in Table 1 .
Table 1
Demographic and Clinical Characteristics of the Studied Patients
|
Variables |
N (%) / Mean ± SD (Range) |
|
Age (years) |
43.88 ± 11.60 (21.00 – 72.00) |
|
Gender (Male) |
64 (64%) |
|
Gender (Female) |
36 (36%) |
|
Male/Female Ratio |
16/9 |
|
Weight (kg) |
87.80 ± 16.39 (27 – 114) |
|
BSA (m²) |
2.00 ± 0.22 (0.97 – 2.26) |
|
Height (cm) |
168.98 ± 8.80 (127 – 181) |
|
Diabetic |
28 (28%) |
|
Non-diabetic |
72 (72%) |
|
Hypertensive |
82 (82%) |
|
Normotensive |
18 (18%) |
|
Smoker |
32 (32%) |
|
Non-smoker |
68 (68%) |
|
Systolic BP (mm Hg) |
145.20 ± 21.55 (90 – 180) |
|
Diastolic BP (mm Hg) |
83.10 ± 10.68 (60 – 100) |
|
Heart rate / minute |
77.68 ± 6.30 (65 – 90) |
|
Onset of dialysis (years) |
3.25 ± 2.84 (0.50 – 14.00) |
|
Frequency of dialysis (frequen-cy/week) |
3.00 ± 0.00 (3.00) |
|
Serum creatinine (mg/dL) |
7.83 ± 2.63 (4.20 - 15.10) |
|
Hemoglobin (g/dL) |
10.69 ± 1.76 (7.60 – 14.70) |
BSA: Body Surface Area, BP: Blood Pressure, SD: Standard Deviation, kg: Kilogram, Milligrams per Deciliter, cm: Centimeter, mm Hg: Millimeters of Mercury, mg/dL: g/dL: Grams per Deciliter.
Table 2 shows the echocardiographic findings of the studied participants.
Diastolic dysfunction was observed in 78% of the patients, with 46% exhibiting grade I diastolic filling, 26% showing grade II diastolic filling, and 6% presenting with grade III diastolic filling.
E/A ratio detected 22 true positive cases and thus 56 false negative cases with a sensitivity of 28.2%. E/e’
Table 2
Echocardiographic findings of the studied participants.
|
Variables |
Echocardiographic Findings |
|
|
Mean ± SD |
Range |
|
|
LVEDD |
51.22 ± 6.72 |
31.00 - 65.00 |
|
LVESD |
33.64 ± 5.31 |
20.00 - 43.00 |
|
IVSd |
11.22 ± 2.29 |
8.00- 18.00 |
|
LVPWd |
11.40 ± 2.16 |
8.00 - 18.00 |
|
EF |
62.50 ± 5.56 |
50.00 - 72.00 |
|
LV mass |
235.58 ± 89.38 |
87.00- 468.00 |
|
LVMI |
118.92 ± 44.53 |
47.00 - 234.00 |
|
E (cm/sec) |
88.13 ± 29.78 |
38.40 - 187.20 |
|
E/A |
1.08 ± 0.45 |
0.50 - 2.90 |
|
DT (msec) |
174.74 ± 36.98 |
105.00 - 250.00 |
|
Lateral E’ (cm/sec) |
10.39 ± 3.56 |
6.00 - 20.00 |
|
E/E’ |
9.41 ± 4.15 |
3.10 - 20.00 |
|
LA diameter |
40.60 ± 5.45 |
30.00 - 55.00 |
|
LA volume index |
26.54 ± 7.49 |
16.00 - 47.00 |
|
TR Vmax |
2.52 ± 0.80 |
0.90 - 3.60 |
LVPWd: Left Ventricular Posterior Wall Thickness in Diastole, LVEDD: Left Ventricular End-Diastolic Diameter, LVESD: Left Ventricular End-Systolic Diameter, LVMI: Left Ventricular Mass Index, IVSd: Interventricular Septal Thickness in Diastole, EF: Ejection Fraction, DT: Deceleration Time, E: Early Diastolic Filling Velocity, E/A: Ratio of Early to Late Diastolic Filling Velocities, E’: Early Diastolic Velocity of Mitral Annulus by Tissue Doppler Imaging, E/E’: Ratio of Early Filling Velocity to Early Mitral Annulus Velocity, LA: Left Atrium, TR Vmax: Maximum Tricuspid Regurgitation Velocity.
ratio detected 58 true positive cases and thus 20 false negative cases with a sensitivity of 74.3%. Comparison of E/A echocardiographic changes and that of E/e’ showed statistical difference in detecting diastolic dysfunction (χ2 = 33.2526 and p = < 0 .0001). Table 4
Regression Summary for diastolic filling in studied patients: R= 0.95 R²= 0.91 Adjusted R²= 0.88 F (26, 73) =28.79 p
We plotted multiple regression analyses for studied patients to conclude the most important predictor of diastolic filling in the study. We found that smoking, DM, Hb, LVPWd, EF, E/A, LA volume index and TR Vmax presented the important determinant of diastolic filling (β = -0.16, 0.15, -0.20, -0.27, -0.25, 0.16, 0.39 & 0.27 and p = 0.002, 0.045, < 0.001, 0.022, < 0.001, 0.039, < 0.001 & < 0.001 respectively). Table 5
DISCUSSION
The primary purpose of this study was to assess the prevalence of diastolic dysfunction in patients undergoing renal replacement therapy, specifically dialysis, and to examine the relationship between echocardiographic and TDI parameters with patient risk factors and hemoglobin levels. A significant finding was that 71% of the study participants had anemia, a much higher prevalence than the 54% reported by Jacob et al. 13 in HFpEF patients. This disparity is likely due to the study population, as our participants were exclusively ESRD patients receiving regular dialysis, which
Table 3
Correlation between echocardiographic Findings and the risk factors of the studied participants.
|
Age |
Smoking |
DM |
HTN |
Onset of dialysis |
ECG |
Hb |
Serum creatinine |
||
|
LVEDD |
r |
0.23 |
0.27 |
0.11 |
0.24 |
0.01 |
-0.04 |
-0.16 |
-0.21 |
|
P |
0.021 |
0.00 6 |
0.276 |
0.016 |
0.921 |
0.693 |
0.111 |
0 .03 6 |
|
|
LVESD |
r |
0.15 |
0.21 |
0.09 |
0.21 |
-0.06 |
-0.15 |
-0.12 |
-0.14 |
|
P |
0.298 |
0 .03 6 |
0.373 |
0 .036 |
0.553 |
0.693 |
0.111 |
0.165 |
|
|
IVS d |
r |
0.17 |
0.10 |
0.14 |
0.11 |
-0.03 |
0.23 |
-0.17 |
0.14 |
|
P |
0.091 |
0.322 |
0.165 |
0.276 |
0.767 |
0.021 |
0.091 |
0.165 |
|
|
LVPW d |
r |
-0.07 |
0.23 |
0.03 |
-0.01 |
0.09 |
0.22 |
-0.01 |
0.14 |
|
P |
0.489 |
0.021 |
0.767 |
0.921 |
0.373 |
0.028 |
0.921 |
0.165 |
|
|
EF |
r |
0.23 |
-0.12 |
-0.02 |
0.09 |
-0.01 |
0.15 |
0.01 |
-0.23 |
|
P |
0.021 |
0.111 |
0.843 |
0.373 |
0.921 |
0.693 |
0.921 |
0.021 |
|
|
LV mas s |
r |
0.14 |
0.30 |
0.02 |
0.09 |
0.15 |
0.26 |
-0.14 |
-0.03 |
|
P |
0.165 |
0.002 |
0.843 |
0.373 |
0.693 |
0.009 |
0.165 |
0.767 |
|
|
Age |
Smoking |
DM |
HTN |
Onset of dialysis |
ECG |
Hb |
Serum creatinine |
||
|
LVMI |
r |
0.05 |
0.26 |
-0.05 |
0.10 |
0.28 |
0.31 |
-0.11 |
-0.03 |
|
P |
0.621 |
0.009 |
0.621 |
0.322 |
0.005 |
0.002 |
0.276 |
0.767 |
|
|
E (cm/sec ) |
r |
-0.11 |
-0.06 |
0.25 |
0.31 |
0.18 |
0.07 |
-0.25 |
-0.06 |
|
P |
0.276 |
0.553 |
0.012 |
0.002 |
0.073 |
0.489 |
0.012 |
0.553 |
|
|
E/ A |
r |
-0.26 |
-0.22 |
-0.01 |
0.19 |
0.01 |
-0.17 |
-0.37 |
-0.02 |
|
P |
0.009 |
0.028 |
0.921 |
0.05 |
0.921 |
0.091 |
< 0.001 |
0.843 |
|
|
DT (msec ) |
r |
0.16 |
0.06 |
0.14 |
0.16 |
-0.14 |
0.02 |
-0.05 |
-0.12 |
|
P |
0.111 |
0.553 |
0.165 |
0.111 |
0.165 |
0.843 |
0.621 |
0.111 |
|
|
Lateral E’ (cm/sec ) |
r |
-0.0004 |
0.27 |
-0.005 |
-0.13 |
0.04 |
0.01 |
0.12 |
-0.01 |
|
P |
0.997 |
0.00 6 |
0.961 |
0.197 |
0.693 |
0.921 |
0.111 |
0.921 |
|
|
E/E ’ |
r |
-0.06 |
-0.28 |
0.08 |
0.35 |
0.07 |
0.06 |
-0.29 |
-0.06 |
|
P |
0.553 |
0.005 |
0 .429 |
< 0.001 |
0.489 |
0.553 |
0.003 |
0.553 |
|
|
LA diamete r |
r |
0.35 |
0.11 |
-0.03 |
0.13 |
-0.09 |
-0.06 |
-0.12 |
-0.15 |
|
P |
< 0.001 |
0.276 |
0.767 |
0.197 |
0.373 |
0.553 |
0.111 |
0.693 |
|
|
LA volume indexes |
r |
0.27 |
-0.02 |
0.05 |
0.15 |
-0.10 |
0.07 |
-0.23 |
0.07 |
|
P |
0.006 |
0.843 |
0.621 |
0.693 |
0.322 |
0.489 |
0.02 1 |
0.489 |
|
|
TR Vmax |
r |
-0.17 |
-0.12 |
-0.05 |
0.11 |
-0.01 |
-0.24 |
-0.31 |
-0.30 |
|
P |
0.091 |
0.111 |
0.621 |
0.276 |
0.921 |
0.016 |
0.002 |
0.002 |
|
|
Diastolic filling |
r |
0.04 |
-0.27 |
0.1 |
0.29 |
-0.04 |
-0.12 |
-0.53 |
-0.09 |
|
P |
0.693 |
0.006 |
0.921 |
0.003 |
0.693 |
0.111 |
< 0.001 |
0.373 |
|
Comparison between E/A and E/e’ regarding their sensitivity in detecting diastolic dysfunction
Table 4
Significance between E/A and E/e’
|
E/A |
E/e’ |
χ2 |
P |
||
|
True Positive |
False negative |
True Positive |
False Negative |
33.253 |
< 0 .0001 |
|
22 |
56 |
58 |
20 |
||
E/A: Ratio of Early to Late Diastolic Filling Velocities, E/e’: Ratio of Early Filling Velocity to Early Mitral Annulus Velocity, χ²: ChiSquare.
Table 5
Multiple Regression Analysis of Different Factors Affecting diastolic filling in all studied patients.
|
Β |
B |
t (73) |
p-value |
|
|
Intercept |
4.006 |
1.58 |
0.119 |
|
|
Age |
-0.10 |
-0.01 |
-1.13 |
0.261 |
|
Sex |
-0.06 |
-0.10 |
-1.07 |
0.290 |
|
Smokin g |
-0.16 |
-0.30 |
-3.16 |
0.002 |
|
DM |
0.15 |
0.28 |
2.04 |
0.045 |
|
HTN |
0.05 |
0.10 |
0.97 |
0.337 |
|
Onset of dialysis |
-0.04 |
-0.01 |
-0.75 |
0.456 |
Regarding diastolic function, 74% of the patients exhibited varying degrees of diastolic dysfunction: 46% demonstrated grade I filling, 26% grade II, and 6% grade III. In contrast to Malik et al. 14, who reported diastolic dysfunction in 54% of their research cohort (including indeterminate cases), our data indicate a higher prevalence, possibly due to the exclusion of indeterminate patients from our analysis. The elevated prevalence of grade I dysfunction in our study underscores the need for early detection and intervention in this patient population.
The relationship between ESRD and HFpEF was further explored using the E/E’ ratio, which is recognized as a highly reliable noninvasive marker of elevat- 92 | Cardiometry | Issue 33. November 2024
ed LV filling pressure and final diastolic pressure. Our analysis revealed a significant increase in the E/E’ ratio across the patient cohort, indicating elevated LV filling pressures. This finding is consistent with the study by Masugata et al. 15, which demonstrated increased E/E’ ratios in CKD patients with reduced GFR. Bar-berato et al. 16 emphasized the notable independence of TDI-derived velocities from preload, making TDI a particularly valuable tool for distinguishing pseudonormalization from true diastolic dysfunction in dialysis patients.
TDI was instrumental in identifying diastolic dysfunction in this study. Using the diagnostic criteria established by the American Society of Echocardiography, we determined that 78% of the patients had diastolic dysfunction. The E/E’ ratio had a sensitivity of 74.3% in detecting diastolic dysfunction, far exceeding the E/A ratio, which had a sensitivity of just 28.2%. This difference was statistically significant (χ² = 33.2526, p < 0.0001), reinforcing the E/E’ ratio as a superior diagnostic tool in this patient population.
Multivariate regression analysis identified several key variables influencing diastolic filling in the study cohort, including smoking, DM, hemoglobin levels, LVPWd, EF, E/A ratio, LA volume index, and tricuspid regurgitant velocity (TR Vmax). All of these parameters were significantly associated with diastolic filling, confirming their importance in predicting diastolic dysfunction in ESRD patients. In contrast, Masugata et al. 15 examined the relationship between echocardiographic parameters and GFR across different stages of CKD. Our study specifically evaluated the impact of these factors on LV diastolic filling in ESRD patients undergoing regular dialysis, thereby enhancing the understanding of these correlations in this high-risk population.
Our results revealed a high incidence of LV diastolic dysfunction, with 78% of ESRD patients exhibiting relaxation abnormalities. Diastolic dysfunction progresses through several stages, beginning with an inversion of the early-to-atrial velocity ratio, followed by pseudonormalization, and eventually developing into a restrictive filling pattern. Patients with advanced CKD are more likely to display pseudonormal or restrictive patterns of LV filling. These findings are consistent with studies reporting a 50-65% prevalence of diastolic dysfunction in CKD patients, including those on dialysis and renal transplant recipients. Moreover, research has shown that diastolic dysfunction often precedes systolic dysfunction and accounts for 3040% of HF cases.
Diastolic HF is primarily diagnosed through clinical symptoms and echocardiographic assessments of systolic function and diastolic dysfunction. The E/E’ ratio is a crucial parameter for evaluating LV filling pressures and diagnosing diastolic HF, particularly in hypertensive patients. Given that HF affects more than 50% of individuals with renal failure, the timely recognition and treatment of HF in patients with CKD and ESRD are essential for improving outcomes.
This study highlights the complex, interdependent relationship between HF and kidney dysfunction. Even a modest decline in renal function is associated with a poorer prognosis in HF patients, while reduced renal perfusion further impairs cardiac function, creating a harmful cycle. The interplay between cardiac and renal insufficiency is a pivotal factor in the progression of HF, recurrent decompensation, and hospitalization in CKD patients. Addressing these intricate relationships is crucial for optimizing the therapeutic management of CKD patients with diastolic dysfunction.
Study Limitations
A key limitation of this study is the relatively small sample size, with all participants being recruited from a single center, which introduces the potential for selection bias. A larger, more diverse cohort is needed to better reflect the true prevalence of diastolic dysfunction in CKD patients. Additionally, our study focused exclusively on ESRD patients with preserved LVEF, meaning the findings may underestimate the overall impact. The absence of healthy controls is another limitation, restricting the applicability of our results to those with normal ejection fractions. We did not gather data on individuals with reduced ejection fractions. Furthermore, TDI parameters were collected from a single point at the lateral corner of the mitral annulus, without measuring annular velocities from multiple locations, which could affect the comprehensiveness of our assessment.
CONCLUSION
Cardiac abnormalities are common in ESRD patients, particularly those with hypertension and anemia. LVH is the most frequent, followed by diastolic dysfunction, and both are more pronounced in hypertensive or anemic cases. Echocardiography, a noninvasive and cost-effective tool, is crucial for early detection, risk stratification, and prevention. Regular echocardiographic screening with tissue Doppler is recommended for asymptomatic ESRD patients, especially those with hypertension or anemia, due to its strong prognostic value.
Funding
There is not to be declared.
Conflict of interest
The author(s) declare no conflicts of interst.
Список литературы Prevalence of left ventricular diastolic dysfunction by tissue Doppler imaging in patients with end stage renal disease undergoing regular dialysis with preserved systolic function
- Francis A, et al. Chronic kidney disease and the global public health agenda: an international consensus. Nature Reviews Nephrology. 2024/07/01 2024; 20(7):473-85. doi:10.1038/s41581-024-00820-6
- Saeed D, et al. Navigating the Crossroads: Understanding the Link Between Chronic Kidney Disease and Cardiovascular Health. Cureus. Dec 2023; 15(12):e51362. doi:10.7759/cureus.51362
- Echefu G, et al. Pathophysiological concepts and screening of cardiovascular disease in dialysis patients. Front Nephrol. 2023;3:1198560. doi:10.3389/fneph.2023.1198560
- Law JP, et al. Hypertension and cardiomyopathy associated with chronic kidney disease: epidemiology, pathogenesis and treatment considerations. J Hum Hypertens. Jan 2023;37(1):1-19. doi:10.1038/s41371-022-00751-4
- Di Lullo L, et al. Left Ventricular Hypertrophy in Chronic Kidney Disease Patients: From Pathophysiology to Treatment. Cardiorenal Med. Oct 2015;5(4):254-66. doi:10.1159/000435838
- Patel SK, et al. Left ventricular hypertrophy in experimental chronic kidney disease is associated with reduced expression of cardiac Kruppel-like factor 15. BMC Nephrol. Jul 3 2018;19(1):159. doi:10.1186/s12882-018-0955-9
- Rroji M, Figurek A, Spasovski G. Should We Consider the Cardiovascular System While Evaluating CKD-MBD? Toxins (Basel). Feb 25 2020;12(3) doi:10.3390/toxins12030140
- Brandenburg V, Ketteler M. Vitamin D and Secondary Hyperparathyroidism in Chronic Kidney Disease: A Critical Appraisal of the Past, Present, and the Future. Nutrients. Jul 22 2022; 14(15)doi:10.3390/nu14153009
- Kim JS, Hwang HS. Vascular Calcification in Chronic Kidney Disease: Distinct Features of Pathogenesis and Clinical Implication. Korean Circ J. Dec 2021; 51(12):961-982. doi:10.4070/kcj.2021.0995
- Habas E, et al. Secondary Hyperparathyroidism in Chronic Kidney Disease: Pathophysiology and Management. Cureus. Jul 2021;13(7):e16388. doi:10.7759/cureus.16388
- Health NIf, Excellence C. Surveillance report 2017—chronic kidney disease (stage 4 or 5): management of hyperphosphataemia (2013) NICE guideline CG157, chronic kidney disease in adults: assessment and management (2014) NICE guideline CG182 and chronic kidney disease: managing anaemia (2015) NICE guideline NG8. National Institute for Health and Clinical Excellence London; 2017.
- Nagueh SF, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. Apr 2016;29(4):277-314. doi:10.1016/j.echo.2016.01.011
- Burns JA, et al. Lack of Association Between Anemia and Intrinsic Left Ventricular Diastolic Function or Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction. Am J Cardiol. Oct 15 2018; 122(8): 1359-65. doi:10.1016/j.amjcard.2018.06.045
- Malík J, et al. Diastolic dysfunction in asymptomatic hemodialysis patients in the light of the current echocardiographic guidelines. The International Journal of Cardiovascular Imaging. 02/01 2019; 35:1-5. doi:10.1007/s10554-019-01564-2
- Masugata H, et al. Echocardiographic assessment of the cardio-renal connection: is left ventricular hypertrophy or diastolic function more closely correlated with estimated glomerular filtration rate in patients with cardiovascular risk factors? Clin Exp Hypertens. Jan 2010; 32(2): 113-20. doi:10.3109/10641960902993145
- Barberato SH, Pecoits Filho R. Prognostic value of left atrial volume index in hemodialysis patients. Arq Bras Cardiol. Jun 2007; 88(6): 643-50. doi:10.1590/s0066-782x2007000600004


