Research of 2-thiouracil derivatives by X-ray method
Автор: Frolova T.V., Kim D.G., Sharutin V.V., Osheko K.Yu.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Органическая химия
Статья в выпуске: 3 т.7, 2015 года.
Бесплатный доступ
2-Propargylthio-6-methyl-5-ethyl-4(3H)-pyrimidinone has been synthesized by alkylation of S-sodium salt of 6-methyl-5-ethyl-2-thiouracil with propargyl bromide. It has been found by X-ray method that S-derivatives of 2-thiouracil are in the tautomeric form with the proton at the nitrogen atom N3. The substitute at the sulfur atom is within the plane of pyrimidine ring at the angle 59-84°. The 2-alkylthio-4(3H)-pyrimidinones molecules are combined in dimers with formation of two intermolecular hydrogen bonds.
2-prenylthio-6-trifluoromethyl-4(3h)-pyrimidinone, cis-2-(3-chloroallyl)thio-6-trifluoromethyl-4(3h)-pyrimidinone, 2-allylthio-6-methyl-4(3h)-pyrimidinone, 2- propargylthio-6-methyl-5-ethyl-4(3h)-pyrimidinone, alkylation, propargyl bromide, tautomerism, molecular structure, x-ray analysis
Короткий адрес: https://sciup.org/147160315
IDR: 147160315 | УДК: 547.854.83+548.312.5
Текст научной статьи Research of 2-thiouracil derivatives by X-ray method
It is known that alkylation of 6-methyl-2-thiouracil and 6-trifluoromethyl-2-thiouracil occurs at the sulfur atom [1]. At the same time information about alkylation of 6-methyl-5-ethyl-2-thiouracil by propargyl bromide has not been discussed in literature. The aim of the present study is the synthesis of 2-propargylthio-6-methyl-5-ethyl-4(3 H )-pyrimidinone and the investigation of its structure and other 2-thiouracil S-derivatives by X-ray analysis.
ExperimentalSynthesis of 2-propargylthio-6-methyl-5-ethyl-4(3H)-pyrimidinone (1)
Propargyl bromide (0.9 mL, 0.001 mol) and NaOH (0.04 g, 0.001 mol) were added to the S-sodium salt of 6-methyl-5-ethyl-2-thiouracil solution (0.912 g, 0.001 mol) in 20 mL of water. The reaction mixture was stirred with a magnetic stirrer for 2 h. Mixture was neutralized by the addition of acetic acid, the obtained precipitate was filtered off, washed with water, dried and recrystallized from hexane. The product yield: 0.154 g (55%), T m =162°С, R f =0.55(ethyl acetate:hexane 1:3). Crystals for X-ray analysis were obtained from the isooctane – acetone (3:1) solution.
Compounds 2–4 were obtained by the interaction between S-sodium salt of 6-methyl- and 6-trifluoromethyl-2-thiouracil with allyl bromide, prenyl chloride and 1,3-dichloropropene by the method [2].
The X-ray analysis of the crystals of compounds 1–4 was performed on the automatic four-circle Bruker D8 Quest diffractometer (Mo K α -radiation, λ = 0.71073 Å, graphite monochromator).
The data collection and editing, as well as the refinement of unit cell parameters and the absorption accounting were carried out using SMART and SAINT Plus program packages [3]. All calculations for the structure determination and refinement were carried out using the SHELXTL/PC [4] program packages. The structures of compounds 1–4 were determined by the direct method and refined by leastsquares method calculations in anisotropic approximation for non-hydrogen atoms. Selected crystallographic data and structure refinement results are given in Table 1.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (№ 998573, 1061098, 1061104, 1028131;
; .
Table 1
Crystallographic data and the experimental and structure refinement parameters for compounds 1–4
|
Parameter |
Value |
|||
|
1 |
2 |
3 |
4 |
|
|
Empirical formula |
C 10 H 12 N 2 OS |
C 8 H 10 N 2 OSH 10 |
C 10 H 11 N 2 SF 3 O |
C 8 H 6 N 2 OSClF 3 |
|
Formula weight |
208.28 |
182.24 |
264.27 |
270.66 |
|
Т , К |
273.15 |
273.15 |
295.0(2) |
295.0(2) |
|
Crystal system |
Monoclinic |
Triclinic |
Monoclinic |
Monoclinic |
|
Space group |
P2 1 /c |
P-1 |
P2 1 /n |
P2 1 /n |
|
a , Å |
9.8551(18) |
4.6742(2) |
12.675(17) |
12.080(11) |
|
b , Å |
21.875(3) |
9.5013(4) |
7.156(9) |
7.43(2) |
|
c, Å |
4.8936(8) |
11.4968(5) |
13.644(17) |
12.650(16) |
|
α , deg |
90.00 |
113.905(2) |
90.00 |
90.00 |
|
β, deg |
93.569(4) |
99.243(2) |
95.10(6) |
91.56(9) |
|
γ , deg |
90.00 |
92.850(2) |
90.00 |
90.00 |
|
V , Å3 |
1052.9(3) |
456.98(3) |
1233(3) |
1134(4) |
|
Z |
4 |
2 |
4 |
4 |
|
ρ (calcd.), g/сm3 |
1.314 |
1.324 |
1.424 |
1.585 |
|
µ , mm – 1 |
0.276 |
0.307 |
0.286 |
0.540 |
|
F (000) |
440.0 |
192.0 |
544.0 |
544.0 |
|
Crystal size, mm |
0.24 × 0.09 × 0.025 |
0.53 × 0.25 × 0.13 |
0.61 × 0.2 × 0.12 |
0.6 × 0.27 × 0.25 |
|
θ Range of data collection, deg |
6.96 to 52.78° |
7.32 to 52.84° |
4.6 to 57.04° |
4.6 to 42.9° |
|
Range of refraction indices |
– 12 ≤ h ≤ 12, – 27 ≤ k ≤ 27, – 6 ≤ l ≤ 6 |
– 5 ≤ h ≤ 5, – 11 ≤ k ≤ 11, – 14 ≤ l ≤ 14 |
– 8 ≤ h ≤ 16, – 9 ≤ k ≤ 9, – 18 ≤ l ≤ 18 |
– 12 ≤ h ≤ 12, – 7 ≤ k ≤ 7, – 9 ≤ l ≤ 13 |
|
Measured reflections |
14398 |
10518 |
11916 |
3767 |
|
Independent reflections |
2138 |
1868 |
3107 |
1248 |
|
R int |
0.1804 |
0.0233 |
0.0362 |
0.0328 |
|
GOOF |
0.996 |
0.984 |
1.017 |
1.088 |
|
R factors for F2 > 2 σ (F2) |
R 1 = 0.0613, wR 2 = 0.1025 |
R 1 = 0.0347, wR 2 = 0.1123 |
R 1 = 0.0764, wR 2 = 0.2049 |
R 1 = 0.0726, wR 2 = 0.2012 |
|
R factors for all reflections |
R 1 = 0.1481, wR 2 = 0.1283 |
R 1 = 0.0458, wR 2 = 0.1233 |
R 1 = 0.1189, wR 2 = 0.2376 |
R 1 = 0.0987, wR 2 = 0.2425 |
|
Residual electron density (min/max), e /Å3 |
0.22/ – 0.21 |
0.21/ – 0.20 |
0.93/ – 0.24 |
0.72/ – 0.25 |
Results and Discussion
We are the first to find that propargyl bromide is carried out pyrimidinone ( 1 ).
alkylation of S-sodium salt of 6-methyl-5-ethyl-2-thiouracil by with the synthesis of 2-propargylthio-6-methyl-5-ethyl-4(3 H )-
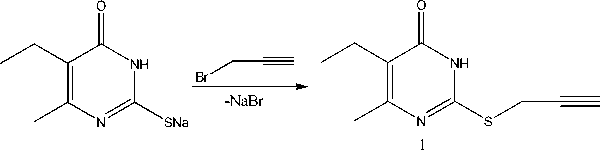
2-Allylthio-6-methyl-4(3H)-pyrimidinone (2), 2-prenylthio-6-trifluoromethyl-4(3H)-pyrimidinone (3) and cis-2-(3-chloroallyl)thio-6-trifluoromethyl-4(3H)-pyrimidinone (4) are synthesized by the method [2]. Note that alkylation of 6-trifluoromethyl-2-thiouracil by 1,3-dichloropropene is carried out with the formation of mixture of cis- and trans-isomers. The monocrystal of cis-isomer has been isolated mechanically after recrystallization from hexane.
It has been found by X-ray method that derivatives at the sulfur atom in sulfides 1 – 4 (Fig. 1 – 4) are almost in the perpendicular plane to pyrimidine ring at the angle 72 – 84°. Exception is chloroallylsulfide 4 , in which the chloroallyl fragment is at the angle that equals 59° for pyrimidine ring.
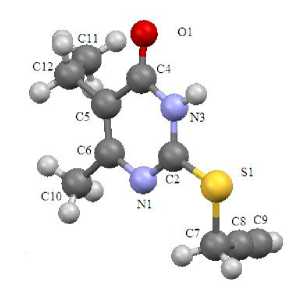
Fig. 1. The structure of compound 1
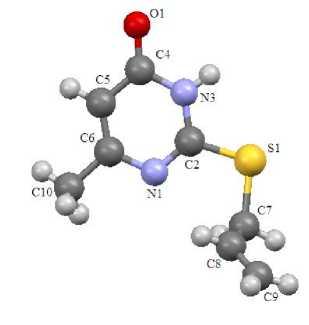
Fig. 2. The structure of compound 2
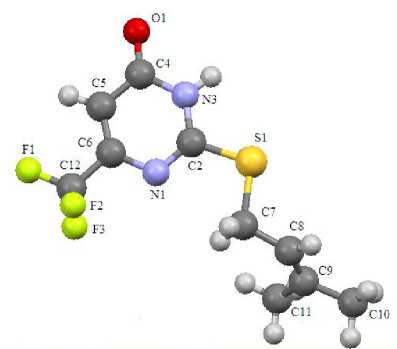
Fig. 3. The structure of compound 3
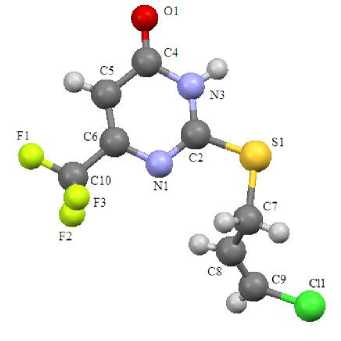
Fig. 4. The structure of compound 4
Sulfides 1 – 4 have standard geometry. Particularly there is noticeable asymmetry of the C – S bond lengths, which is the result of heterocycle effect (C(2)–S(1)= 1.727 – 1.759 Å, C(7)–S(1)= 1.802 – 1.822 Å). The bond lengths of heterocycles in all analyzed compounds are in the range 1.299 – 1.384 Å. Exception is the bond length С(5)–С(4) 1.420 – 1.442 Å. It is the result of the influence of the electron acceptor oxygen atom at С(4).
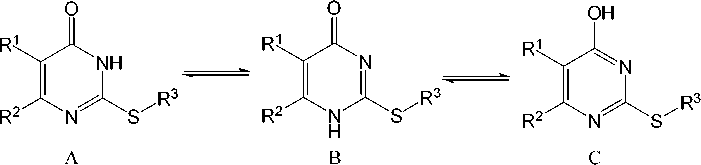
Three tautomeric forms for sulfides 1 – 4 can be presented:
Selected bond lengths in the structures of compounds 1 - 4
Table 2
|
Bond length, Å |
Compound |
|||
|
1 |
2 |
3 |
4 |
|
|
С4 – О1 |
1.242(3) |
1.238(2) |
1.224(4) |
1.229(8) |
|
С2 – S1 |
1.750(3) |
1.759(2) |
1.741(4) |
1.759(7) |
|
С7 – S1 |
1.811(3) |
1.807(2) |
1.822(5) |
1.818(9) |
|
С2 – N3 |
1.353(4) |
1.353(2) |
1.353(4) |
1.347(9) |
|
С6 – N1 |
1.380(4) |
1.379(2) |
1.364(4) |
1.379(9) |
|
С4 – N3 |
1.377(4) |
1.381(2) |
1.384(4) |
1.397(8) |
|
С2 – N1 |
1.292(4) |
1.300(2) |
1.295(4) |
1.313(9) |
|
С5 – С6 |
1.357(4) |
1.358(2) |
1.347(5) |
1.356(10) |
|
С4 – С5 |
1.441(4) |
1.421(2) |
1.420(5) |
1.442(10) |
The С – О bond length of sulfides 1 – 4 corresponds to the carbonyl group (1.224 – 1.242 Å) which is absent in tautomeric form C. The С(2)–N(1) bond length in compounds 1 – 4 is 1.299 – 1.313 Å. It is the result of double bond С=N formation in sulfides, which opposes structure B. Thus, compounds 1 – 4 are in tautomeric form A. It corresponds to the various quantum-chemical calculations and analysis of vibrational spectra in the works [5, 6].
The molecules of sulfides 1 – 4 are combined in dimers due to formation of hydrogen bonds, these dimers are oriented in the following way: the oxygen atom of the first molecule is bonded with the NH group of the second molecule, and the NH group of the first molecule is bonded with the oxygen atom of the second molecule (Fig. 5). The length of hydrogen bonds in compounds 2 and 4 is 1.889 and 1.896 Å. This length in compounds 1 and 3 is equal to 1.921 and 1.923 Å. The dimers are almost in the same plane, and the deviation is not more than 1 – 2°. The dimers of sulfides 1 – 4 are packed in parallel planes due to short contacts (Fig. 6).
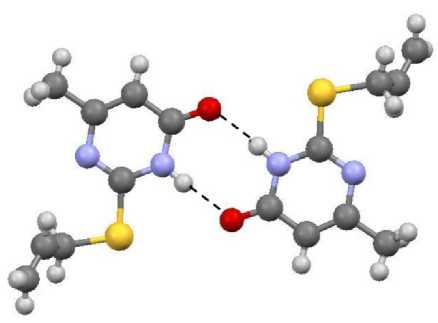
Fig. 5. The structure of compound 2 dimer
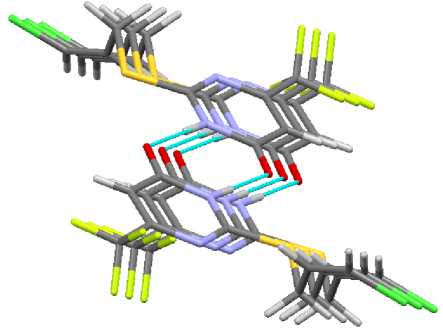
Fig. 6. The packing of molecules in crystal 4
The oxygen of one molecule and the proton of the prenyl fragment methyl group of another molecule form this contact in prenyl sulfide 3 (Fig. 7). The oxygen of one molecule and the proton at the β-carbon atom in the allyl fragment of another molecule form this contact in chloroallyl sulfide 4 (Fig. 8).
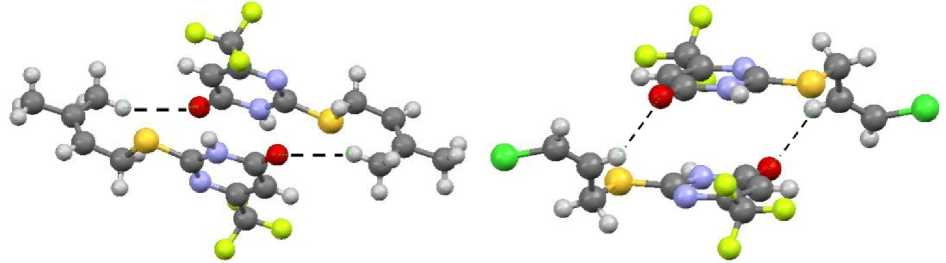
Fig. 7. The packing of molecules in crystal 3
Fig. 8. The packing of molecules in crystal 4
Dimers in propargyl sulfide 1 and allyl sulfide 2 are packed through short contacts between carbonyl carbon atoms О=С … С=О. These lengths are 3.309 and 3.398 Å, respectively (Fig. 9, 10).
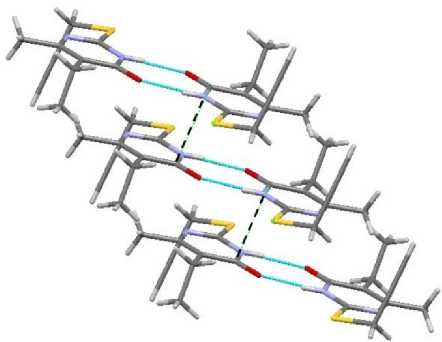
Fig. 9. The short contact in compound 1
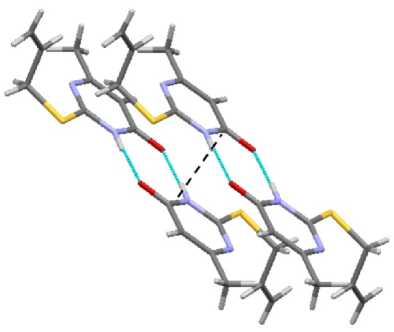
Fig. 10. The short contact in compound 2
In contrast to sulfides 3 and 4 , compounds 1 and 2 have short contacts between sulfur atoms, 3.321 and 3.409 Å, respectively (Fig. 11). It makes the packing of dimers by stacks possible (Fig. 12).
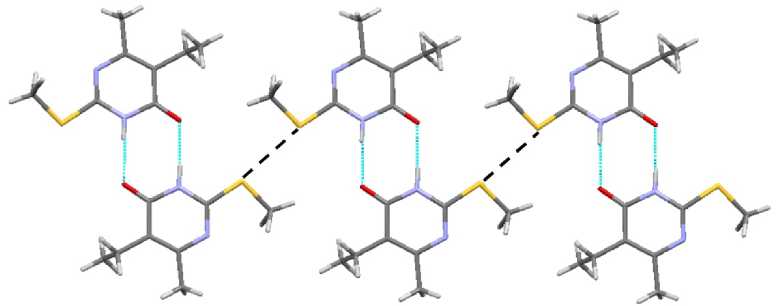
Fig. 11. The short contact in compound 1
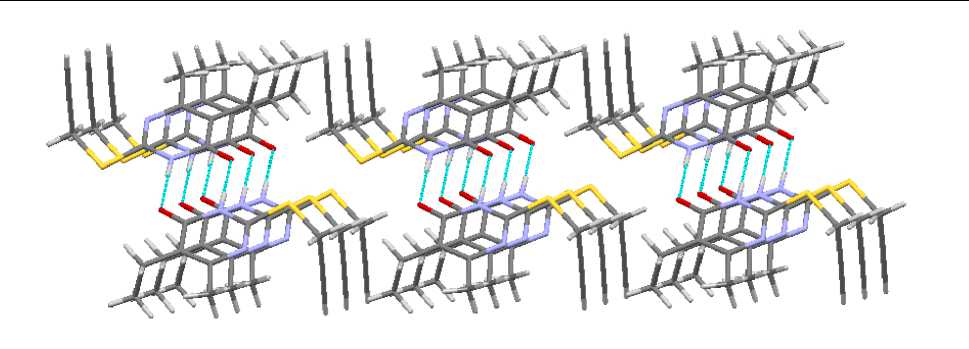
Fig. 12. The packing of molecules in crystal compound 2
In spite of the differences, the packing of compounds 2 – 4 is similar; it is presented on figures 13 and 14. Only in the case of allyl sulfide 1 the packing has two perpendicular directions (Fig. 13), while there is only one direction in compounds 3 and 4 (Fig. 14).
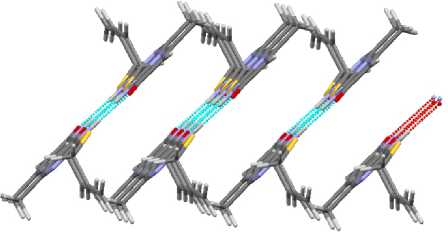
Fig. 13. The packing of molecules in crystal 2
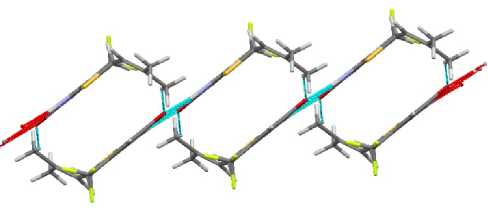
Fig. 14. The packing of molecules in crystal 3
In contrast with crystals 2 – 4 , the stack packing of dimers in the crystal of propargyl sulfide 1 is in perpendicular plane (Fig. 15).
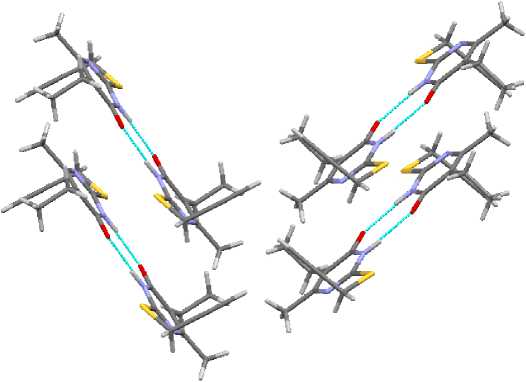
Fig. 15. The packing of molecules in crystal 1
Conclusion
We have found that S-sodium salt of 6-methyl-5-ethyl-2-thiouracil reacts with propargyl bromide to produce 2-propargylthio-6-methyl-5-ethyl-4(3 H )-pyrimidinone. By X-ray method it has been found that S-derivatives of 2-thiouracil are in the tautomeric form with the proton at the nitrogen atom N3 . The substitute group at the sulfur atom is placed at the angle 59–84° with the plane of pyrimidine ring. The 2-alkylthio-4(3 H )-pyrimidinone molecules are combined in dimers with formation of two intermolecular hydrogen bonds.
Список литературы Research of 2-thiouracil derivatives by X-ray method
- Сливка, H.Ю. Галогенциклизация замещенных 2-(алкенилтио)-пиримидин-6-онов/Н.Ю. Сливка, Ю.И. Геваза, В.И. Станинец//Химия гетероциклических соединений. -2004. -№ 5. -С. 776-783.
- Фролова, Т.В. Синтез и исследование S-аллильных производных 2-тиоурацилов/Т.В. Фролова, Д.Г. Ким, П.А. Слепухин//Вестник ЮУрГУ. Серия «Химия». -2010. -Вып. 3. -№. 11. -С. 9-15.
- Bruker (2000) SMART. Bruker Molecular Analysis Research Tool, Versions 5.625 Bruker AXS, Madison, Wisconsin, USA.
- Bruker (2000) SAINTPlus Data Reduction and Correction Program Versions 6.02a, Bruker AXS, Madison, Wisconsin, USA.
- Тен, Г.Н. Определение таутомерных структур тиозамещенных урацила методом ИК и РКР спектроскопии/Г.Н. Тен, Т.Г. Бурова, В.И. Баранов//Журнал структурной химии. -2007. -Т. 48. -№ 3. -С. 492-500.
- Расчет и анализ структуры и колебательных спектров таутомера урацила/Г.Н. Тен, В.В. Нечаев, Р.С. Щербаков, В.И. Баранов//Журнал структурной химии. -2010. -Т. 51. -№ 1. -С. 38-45.


