Research of camel shanks proteinhydrolyzates by electrophoregram
Автор: Satayeva Zh. I., Tayeva A. M.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Техника и технологии
Статья в выпуске: 3 (128), 2020 года.
Бесплатный доступ
This research aims to study the degree of hydrolysis, determining the nature of protein hydrolyzates, which determine their size and molecular weight by the method of electrophoregram. In this research, a camel pancreas suspension was used to hydrolyze proteins from camel shanks. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to monitor the distribution of proteins and evaluate their molecular weights at different incubation times. Electrophoregram processing using the BioCapt program (Vilber Lourmat, France) determines the nature of the hydrolysis of protein and peptide profiles among hydrolyzates and the hydrolysis time for 8 hours shows the most significant accumulation of low molecular weight compounds with a molecular mass of
Amel shanks, pancreas, electrophoregram, protein hydrolyzates, low molecular weight peptides
Короткий адрес: https://sciup.org/140250907
IDR: 140250907 | УДК: 637.69 | DOI: 10.48184/2304-568X-2020-3-67-73
Текст научной статьи Research of camel shanks proteinhydrolyzates by electrophoregram
The growing well-being of the population, rapid growth, and urbanization require improved use of existing protein sources along with the development of new and sustainable food production. World protein demand is expected to double by 2050. This is due to the growing recognition of the critical role of protein in healthy eating in general, and especially for children and a growing elderly population. Meat products are essential sources of protein in the human diet and contain essential amino acids, minerals, and vitamins [1].
Proteins, components necessary for all organisms, are integrated into cellular structures and perform certain functions, as in the case of hormones, antibodies, and enzymes [2]. Secondary products of fish, livestock and poultry processed are a source of protein and other vital nutrients with potential biological active properties, suitable for further processing and improving the value of other products. These include heads, bones, carcasses, blood, skin, viscera, hooves, and feathers. There are many possible solutions to extract valuable nutrients from these substances, and one of the most effective and promising is to obtain a protein hydrolyzate.
Recent scientific evidence suggests that dietary proteins not only serve as nutrients but can also modulate the physiological functions of the body [3]. Protein hydrolyzates of meat byproducts represent an exciting alternative to soy flour, since there are no nutritional factors or allergenic proteins, and large amounts of all essential amino acids are present [4].
Protein hydrolysates can be obtained by hydrolysis of meat products or secondary raw materials under the action of proteolytic enzymes or chemical agents. These hydrolyzates are composed of protein fragments: peptides of various sizes; poly-, three- and dipeptides and free amino acids, which are effectively absorbed in the intestine, and also significantly affect the taste of food [5]. Enzymatic hydrolysis is preferred among hydrolysis methods and has advantages associated with the internal characteristics of enzymes, such as selectivity for substrates, and processes carried out under milder thermal conditions [6, 7].
During enzymatic hydrolysis, the molecular weight of intrinsic proteins and peptides decreases, and the number of ionized groups increases, which leads to the appearance of new peptides that are less and more soluble in water. Enzymatic hydrolysis can be carried out either by using endogenous enzymes that are found naturally in the substrate or by adding commercially available exogenous enzymes [8].
The hydrolysis process involves several variables; specificity and activity of the enzyme, the ratio of the enzyme/substrate, pH, temperature, time, and the interaction between the nutrients present in the feed during hydrolysis [6].
Obtaining protein hydrolyzates is a promising alternative to add value to meat products. Also, protein hydrolyzates can improve and change the functional, physicochemical, and sensory properties of meat products [9].
Objects and methods of research
Camel shanks, samples of liquid and dry hydrolyzate were the objects of research.
The following techniques were used in the research process:
-
- Determination of protein by the Bradford method;
-
- The fractional composition of protein hydrolyzates was determined on an electrophoregram BIO-RAD Consort EV265;
-
- Electrophoregrams of the studied samples were analyzed on a densitometer. Electrophoregram processing was done using the BioCapt program (VilberLourmat, France).
The collagen hydrolyzate of camel shanks was obtained by the enzymatic method. The homogenate of the pancreas of camel and cattle was used as an enzyme-containing raw material.
Before hydrolysis, purified, crushed onto disks weighing 50 g and mixed with distilled water in a ratio of 1:2 were decreased in a thermostated glass at a temperature of 95-98°C for 50-55 minutes in a water bath. After cooling, the released fat was separated - 2.3-2.8% of the mass of camel shanks.
To obtain a suspension, the camel’s pancreas was crushed on a top with a hole diameter of 2-3 mm, homogenized with distilled water at an enzyme: substrate (hydro module) ratio of 1:0.5, then ethanol in an amount of 2% was added to the resulting suspension as a preservative.
Protein isolates were hydrolyzed with a pancreatic suspension at an enzyme: substrate ratio (protein ratio) of 1:15. Thermostatic was carried out at a temperature of 45°C and a pH of 7.0 for 4, 5, 6, 7, 8, 9 hours until the proteins were completely dissolved. To inactivate the enzyme complexes and thermocoagulation the extra protein at the end of the hydrolysis, the obtained substrate enzyme complexes were cooked at a temperature of 90±2°С for 20 minutes with further cooling in ice water to 0°С. The crude hydrolyzate consists of three phases: aqueous, solid, and fat. The solid phase contains bones and insoluble proteins. The fat phase is 0.2-0.5% by weight of the original fat. The aqueous phase is a water-soluble protein hydrolyzate, which is then further processed.
The biological activity of the hydrolyzate depends on the size of the particles and their solubility. The biological and nutritional properties of protein hydrolyzates are determined by the average molecular weight [9, 10, 11].
Determination of protein by the method of Bradford. 12 μl was taken from the fresh hydrolyzate, dried in a thermostat, or using freeze-drying, then 80 μl of urea was added to the precipitate, buffer for diluting samples with 2-mercaptoethanol 30 μl, after stirring, was kept in a thermostat at 95 degrees 45 min. Further, 10 μl of a fresh sample was used for electrophoresis. Then electrophoresis was performed on PAGE 12%, and staining was performed according to the Bradford method.
Analysis of proteins by electrophoresis in SDS-PAGE (polyacrylamide gel with sodium dodecyl sulfate). The initial hydrolyzed, soluble and insoluble fractions were analyzed by electrophoregram in 12% SDS-PAGE according to the method of U.K. Laemmlietal using an apparatus for vertical electrophoresis (Bio-Rad, USA). Well-cleaned washed and fat-free glass plates (7x10) were installed using gaskets and clamps. The space between the plates was poured with a solution for a separating gel consisting of 3.5 ml of 30% acrylamide; 3.1 ml of 1% bisacry-lamide; 7.5 ml of 1.5 M Tris-HCl (pH 8.7); 6.5 ml of distilled water; 0.03 ml 10% SDS; 0.001 ml TEMED and 0.01 ml 10% ammonium persulfate. After polymerization of the lower gel, the remaining space is poured into the solution to concentrate the gel. The solution is prepared as follows: 1 ml of 1% bisacrylamide;1 ml of 30% acrylamide is mixed; 5.35 ml of distilled water; 2.5 ml 0.5 M Tris-HCl (pH 6.8); 0.01 ml 10% SDS; 0.05 ml 10% ammonium persulfate and 0.005 ml TEMED. Immediately after pouring the concen- trating gel, a comb was set until complete polymerization.
A sample dilution buffer was prepared as follows: 0.25 ml of 2-mercaptoethanol was added to 0.315 ml of 1M Tris-HCl (pH 6.8); 0.5 ml glycerol, 0.115 ml 10% SDS; 0.05 ml of 0.1% bromophenol blue and distilled water were added to a volume of 5 ml. Then, the obtained samples were diluted in a ratio of 1:1, boiled in a water bath for 3-5 minutes and then cooled.
Electrophoresis was performed at a current of 20 mA per chamber. At the end of the process, the gel was removed from the plates, staining was carried out for 1 hour in a CoumassyR-250 solution, then washed several times with a bleaching solution until the initial background color disappeared.
The liquid protein hydrolyzate was centrifuged at 10,000 G on an Eppendorf Centrifuge 5810 R for 20 minutes. After separation of the solid phase, the supernatant was dried on a NanBei spray dryer. The temperature at the inlet to the dryer ranged from 138 to 141°C and the temperature at the outlet ranged from 58 to 60°C. The yield of dry hydrolyzate was 6.56.8% by weight of camel shanks. The resulting hydrolyzate is a homogeneous finely divided powder of light beige color, with a weak specific odor, readily soluble in water.
Results and their discussion
The degree of hydrolysis determines the nature of hydrolyzates, determining their size and molecular weight. The fractional composition of protein hydrolyzates obtained with the use of the enzyme complex SPL of camel, depending on the duration of hydrolysis, was determined on an electrophoregram BIO-RAD Consort EV265. Electrophoregrams of the studied samples were analyzed on a densitometer. Electrophoregram processing was done using the BioCapt program (Vilber Lourmat, France).
Figure 1 shows the electrophoresis profiles of sodium dodecyl sulfate in polyacrylamide gel (SDS-PAGE) for protein hydrolyzates taken at different incubation times.
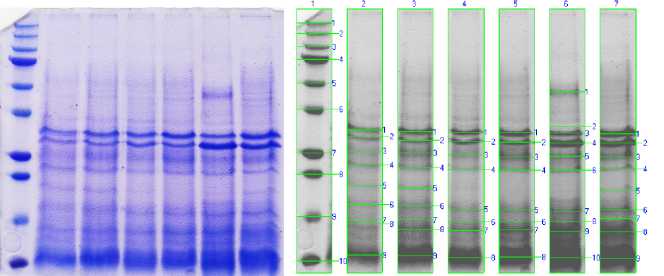
Figure 1 - Electrophoregram separation of the hydrolyzate on a 12% polyacrylamide gel Note:
Lane 1 - Molecular Marker - kDa, (Kaleidoscope™ Prestained Protein Standards, Bio-Rad)
Lane 2 – Sample 1, 4 hourshydrolysis, Lane 3 – Sample 2, 5 hourshydrolysis
Lane 4 – Sample 3, 6 hourshydrolysis, Lane 5 – Sample 4, 7 hourshydrolysis
Lane 6 – Sample 5, 8 hourshydrolysis, Lane 7 - Sample 6, 9 hourshydrolysis
The results indicate that protein hydrolysis continues with an increase in incubation time. But the hydrolysis time for 8 hours shows the most significant accumulation of low molecular weight peptides. Precise profiles of protein hydrolysates show that they have mixed peptides in small amounts. Low molecular weight peptides (2–20 amino acids) are more biologically active than their parent polypeptides/proteins, which are more significant [14]. The process of enzymatic hydrolysis is mainly determined by whether the type of protease involved is endopeptidase or exopeptidase [15]. Endopeptidase and exopeptidase are two types of peptidase enzymes. They cleave peptide bonds in protein molecules. A pancreatic suspension releases these peptidases to maximize protein breakdown. Endopeptidase cleaves peptide bonds within protein molecules and leads to the formation of peptide chains, rather than monomers. Exopeptidase cleaves peptide bonds at the ends and leads to individual amino acids [16]. Thus, the activity of endopeptidase and exopeptidase contributed to the further phased hydrolysis of proteins.
The results of the quantitative determination of protein fractions performed using the BioCapt program (Vilber Lourmat, France) are shown in table 1.
Table 1 - Molecular weight of protein fractions of hydrolyzates, kDa
|
M.W. Values |
Lane 1 |
Lane 2 |
Lane 3 |
Lane 4 |
Lane 5 |
Lane 6 |
Lane 7 |
|
Band 1 |
250.000 |
31.259 |
30.710 |
30.527 |
30.527 |
45.324 |
30.163 |
|
Band 2 |
150.000 |
29.436 |
28.354 |
27.995 |
27.817 |
32.177 |
27.638 |
|
Band 3 |
100.000 |
25.521 |
25.000 |
24.490 |
23.981 |
30.345 |
23.981 |
|
Band 4 |
75.000 |
22.015 |
21.400 |
20.957 |
20.814 |
27.638 |
21.103 |
|
Band 5 |
50.000 |
10.159 |
17.810 |
15.660 |
15.601 |
24.150 |
17.486 |
|
Band 6 |
37.000 |
16.075 |
15.955 |
14.486 |
14.486 |
20.814 |
15.601 |
|
Band 7 |
25.000 |
14.683 |
14.683 |
13.585 |
13.657 |
15.483 |
14.811 |
|
Band 8 |
20.000 |
14.285 |
13.729 |
10.404 |
10.485 |
14.552 |
13.441 |
|
Band 9 |
15.000 |
10.727 |
10.647 |
13.513 |
10.324 |
||
|
Band 10 |
10.000 |
10.404 |
From a comparison of densitograms, we can conclude that the most assimilable low molecular weight peptide fractions with molecular weights of 8–25 kDa of 53.4 and 39.6% (samples 6 and 7) were obtained by enzymatic hydrolysis of the camel pancreatic suspension by the enzyme complex for 8–9 hours respectively.
Qualitative indicators of the obtained dry enzymatic hydrolyzate of proteins from camel shanks are presented in table 2.
Table 2 - Physic-chemical parameters of dry protein hydrolyzate from camel shanks
|
Name of indicator |
Characteristic |
|
Appearance |
Dry product of homogeneous consistency in the form of loose powder, hygroscopic |
|
Color |
Light beige |
|
Smell |
Intrinsic to the raw material of which it is made, odorless |
|
Moisture content, % |
7,0 |
|
Mass fraction of protein,% |
80,0 |
|
Mass fraction of fat,% |
0,15 |
|
Mass fraction of ash,% |
5,0 |
Thus, the resulting protein hydrolyzate containing low molecular weight protein fractions and amino acids with nutritious properties will be used in further work to enrich meat products for the elderly.
Сonclusion
Research results of the nature of protein hydrolysis and peptide profiles among hydrolyzates confirmed that the enzymatic hydrolysis of camel shanks by a camel pancreas suspension was useful for producing low molecular weight compounds with a molecular weight of <20 kDa, which is a favorable result for the potential biological activity of peptides.
In general, the hydrolysis process improves the digestibility of the protein, increases the bioavailability of amino acids, and leads to improved nutritional value. Protein hydrolyzates will be used as alternative ingredients of high nutritional quality with potential use for the production of meat products for herodietic purposes.
Список литературы Research of camel shanks proteinhydrolyzates by electrophoregram
- Boland M.J., Rae A.N., Vereijken J.M., Muwissen P.M., Fisher Ar.R.H., van Boekel M.A.J.S. et al. (2013) The future supply of animal-derived protein for human consumption. Trends Food Sci & Technology 29(1), 2013. - PP. 62-73.
- da Silva, R.R. Bacterial and Fungal Proteolytic Enzymes: Production, Catalysis, and Potential Applications. Applied Biochemistry Biotechnology. 2017 Sep;183(1):1-19.
- Chakrabarti S, Guha S, Majumder K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients. 2018; 10(11):1738. Published 2018 Nov 12.
- Martínez-Alvarez, O., Chamorro, S., Brenes, A. (2015) Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Research International, 73. - PP. 204-212.
- Kristinsson H.G., Rasco B.A. Biochemical and Functional Properties of Atlantic Salmon (Salmo Salar) Muscle Proteins Hydrolyzed with Various Alkaline Proteases. J Agricultural Food Chemistry.- 2000;48(3). - PP. 657-666.
- Dieterich F., Boscolo W.R., Pacheco Bertoldo M.T., et al. Development and characterization of protein hydrolysates originated from animal agro-industrial byproducts. J Dairy Veterinary & Animal Research 2014;1(2):56-61.
- Bhat, Zuhaib & Kumar, Sunil & Bhat, Nina. (2015). Bioactive peptides from the egg: A review. Nutrition & Food Science. 45, 190-212.
- DOI: 10.1108/NFS-10-2014-0088
- Aspevik, T., Oterhals, Å.,Rønning, S.B. et al. Erratum to: Valorization of Proteins from Co- and By-Products from the Fish and Meat Industry. Top Curr Chem (Z) 375, 57 (2017).
- DOI: 10.1007/s41061-017-0146-3
- Gómez-Guillén, M.C.; Giménez, B., López-Caballero M.E., Montero M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloids.- 2011 Dec;25(8). - PP. 1813-1827.
- Choi, D.; Min S.-G.; Jo Y.-J. Functionality of porcine skin hydrolysates produced by hydrothermal processing for the liposomal delivery system. Journal of Food Biochemistry. 2018.- 42(1). - e12464.
- DOI: 10.1111/jfbc.12464
- Khiari Z.; Ndagijimana M.; Betti M. Low molecular weight bioactive peptides derived from the enzymatic hydrolysis of collagen after isoelectric solubilization/precipitation process of turkey by-products. Poultry Science.- 2014. - 93(9). - PP. 2347-2362.
- DOI: 10.3382/ps.2014-03953
- Berdutina A.V. Development of the technology of protein hydrolysates from secondary raw materials of the meat industry. PhD. D. thesis. - M., 2000. - 186 p.
- Surnin E.V. Development of technology for herodietic sausages enriched with biologically active ingredients from pork legs// PhD. D. thesis. - M., 2001. - 137 p.
- Chen N., Yang H., Sun Y., Niu J., Liu S. Purification and identification of antioxidant Peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides. - 2012 Dec;38(2). - PP. 344-349.
- Li B., Chen F., Wang X., Ji B., Wu Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry //Food Chemistry. 2007; 102(4). - PP. 1135-1143.
- "Endopeptidase". Egyptian Journal of Medical Human Genetics, Elsevier. Britannica, edition of the encyclopedia. "Proteolytic enzyme". Encyclopedia Britannica, Encyclopedia Britannica, Inc. May 31. - 2


