Современные методы определения свежести мяса: мини-обзор
Автор: Кулешова О.С., Тихонов С.Л., Тихонова Н.В., Шихалев С.В.
Журнал: Вестник Восточно-Сибирского государственного университета технологий и управления @vestnik-esstu
Рубрика: Пищевые системы (биологические науки, технические науки)
Статья в выпуске: 4 (95), 2024 года.
Бесплатный доступ
Оценка свежести скоропортящихся пищевых продуктов и продовольственного сырья, в частности мяса и мясопродуктов, является важным направлением исследований в области пищевых систем. В основных общепринятых методах определения свежести мяса используют органолептические, микробиологические и физико-химические показатели. Одним из точных методов оценки свежести мяса является анализ изменений, вызванных расщеплением аденозинтрифосфорной кислоты. Для количественного определения производных пурина в пищевых продуктах как одного из показателя свежести используют высокоточные методы, включая высокоэффективную жидкостную хроматографию, капиллярный электрофорез, чрезвычайно эффективную жидкостную хроматографию с массспектрометрией, газовую хроматографию с масс-спектрометрией, которые требуют дорогого оборудования, трудоемки и экономически нецелесообразны. Новым направлением в оценки свежести мяса является применение практических биосенсоров, позволяющих недорого и быстро обнаруживать низкие концентрации анализируемых веществ - показателей свежести продукта. Ключевым требованием является разработка оптимальной чувствительной поверхности, которая может стабилизировать молекулы биологического распознавания и должна быть сопряжена с физическими преобразователями, переводящими полученные данные биологического распознавания в количественный сигнал.
Определение свежести мяса, биосенсоры, протеомика, инструментальные методы, показатели свежести мяса, гиперспектральные технологии
Короткий адрес: https://sciup.org/142243935
IDR: 142243935 | УДК: 637.512.7:637.522(045) | DOI: 10.53980/24131997_2024_4_22
Текст научной статьи Современные методы определения свежести мяса: мини-обзор
Мясо и мясопродукты относятся к скоропортящийся пищевой продукции, поэтому разработка новых, совершенствование известных способов увеличения хранимоспособности и определения свежести мясного сырья и продуктов его переработки являются важным направлением исследований в области пищевых систем. Авторами [1] установлено, что введение в рецептуру мясных рубленых котлет лука угловатого ослабляет развитие окислительной и микробиологической порчи продукта, а внесение 0,2 % высушенного лука угловатого в измельченный говяжий, конский и свиной жиры тормозит процесс окисления и может способствовать удлинению сроков хранения животных жиров [2].
Существует три основных общепринятых метода определения свежести мяса на основе органолептических, микробиологических и физико-химических показателей. Физические и химические тесты или микробиологические эксперименты отличаются высокой точностью, но трудоемки [3]. Сенсорный анализ прост, однако результаты этого типа оценки часто субъективны и не могут представить точные данные для количественного или качественного анализа [4].
В настоящее время применяют инструментальные аналитические технологии, включающие использование электронного носа [5, 6], спектроскопиию [7 - 9], газовую хроматогра-фию-ольфактометрию (GC-O) [10, 11], хроматографию-масс-спектрометрию [12 - 14] и др. Технология GC-IMS сочетает в себе высокую разделяющую способность газовой хроматографии и быстрый отклик спектрометрии ионной подвижности [15]. Но вместе с тем разработка новых методов определения свежести скоропортящейся пищевой продукции остается актуальным направлением научных исследований.
Поэтому целью работы является анализ современных методов, направленных на определение свежести мяса.
Материалы и методы исследования
Для поиска информации по методам оценки свежести мяса был определен запрос по ключевым словам: «свежесть мяса», «определение свежести пищевых продуктов», «показатели свежести мяса», «биосенсоры для определения свежести» и др. Поиск необходимой литературы был ограничен десятью годами. Был проведен ручной поиск статей, на которые имелись ссылки в информативной статье. Для анализа методов определения свежести мяса использованы аннотации, полнотекстовые статьи из журналов открытого и закрытого доступа, размещенных в отечественных и зарубежных базах данных.
Результаты исследований и их обсуждение
За последние десять лет были открыты гиперспектральные методы как новый тип метода экспресс-неразрушающего контроля. Гиперспектральные методы привлекли большое внимание из-за высокой аналитической эффективности, отсутствия необходимости разрушать образец, сложной предварительной обработки и одновременного анализа нескольких показателей. Гиперспектральные технологии широко используются для обнаружения и оценки качества пищевых продуктов и физико-химических показателей, таких как общий летучий основной азот, тиобарбитуровая кислота. Гиперспектральные методы имеют широкий спектр применений и могут быть использованы для определения свежести мяса и мясопродуктов [3–10, 16]. С помощью этих методов можно количественно определять семь метаболитов пуринов по всему пути метаболизма пуринов и разделять сложные смеси на составляющие их компоненты [10].
Перспективным направлением в определении свежести мяса является новый подход с использованием фудомики. Метаболиты, такие как ацетат, лактат, сукцинат, аланин и аминокислоты с разветвленной цепью, могут быть применены в качестве потенциальных биомаркеров для оценки порчи мяса. При применении протеомного метода идентифицируют семь белков (фосфоглюкомутаза-1, пируваткиназа, тяжелая цепь кинезина-1, тропонин Т, десмин и актин) мышцы палтуса (Scophthalmus maximus) выявляют их изменения при хранении. Установлено, что количество вышеуказанных белков достоверно коррелирует с TVB-N, значением K и TVC. Порча мяса напрямую связана с активностью микроорганизмов и эндогенных ферментов, влияющих на метаболизм мясных белков. Таким образом, использование белков и их метаболитов представляет собой вспомогательный подход к оценке свежести мяса. Пептидо-мика, развивающееся направление протеомики, является мощным инструментом для определения свежести и качества мяса. Применение методов пептидомики для идентификации биомаркеров для оценки качества мяса расширилось. Пептиды APAPAPAPPKEEKI и PAPAPAPAPAPAPAPPKE, идентифицированные через 9 месяцев после вяления, могут быть потенциальными маркерами для контроля времени вяления и конечного качества окороков сухого вяления. Идентифицированы четыре эндогенных пептидных маркера методом UHPLC-Q-TOF, которые заметно изменялись со временем хранения, что указывает на их потенциал в качестве индикаторов срока годности. Имеется информация об изменениях эндогенных пептидов во время консервирования свинины и их потенциале в качестве биомаркеров свежести мяса [17–24].
Одним из точных методов оценки свежести мяса является анализ изменений, вызванных расщеплением АТФ, поскольку высокая концентрация катаболитов АТФ хорошо коррелирует с потерей свежести мяса [25]. Однако синтез АТФ происходит из-за катаболизма накопленного гликогена, что приводит к образованию лактата и снижению рН мяса [24]. Со временем уровень АТФ в мышцах будет снижаться, что приведет к дисфункции саркоплазматического белка и активации фермента АТФ, расщеплению АТФ на его метаболиты [26]. Катаболический путь АТФ приводит к образованию метаболитов, таких как аденозиндифосфат (АДФ), аденозинмонофосфат (АМФ), инозинмонофосфат (ИМФ П), инозин (ИНО) и гипоксантин (Нх) [27]. Гипоксантин (Hx) подвергается дальнейшему окислению, в результате чего образуются ксантин (Xa) и мочевая кислота (UA) По содержанию вышеуказанных веществ можно определить свежесть мяса [28]. Концентрация ИМП в мясе может быстро увеличивается, а АТФ, АДФ и АМФ быстро расщепляется [29]. Расщепление АТФ до ИМП придает мясу приятный пикантный вкус [15, 30]. Однако снижение ИМП и образование ИНО и гипоксантина всегда были связаны с потерей свежести. Гипоксантин обычно указывает на свежесть и срок годности мяса [31]. Катаболизм белков, образующих АТФ, в основном вызывается ферментативными реакциями, но следует учитывать, что на гидролиз ИМП, который образует ИНО и гипоксантин, в основном влияют бактерии порчи мяса [32, 33]. АТФ-ассоциированные соединения быстро разлагаются в присутствии ферментов, включая АТФазу, миокиназу, АМФ-дезаминазу, 5'-нуклеотидазу, нуклеозидфосфорилазу и ксантиноксидазу [33 - 35]. Роль каждого фермента в катаболизме АТФ с образованием его метаболитов, по данным [36], показана на рисунке.
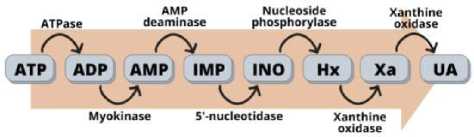
Рисунок – Катаболизм АТФ с соответствующими ферментами, ответственными за окисление каждого соединения [36]
Для количественного определения производных пурина в пищевых продуктах как одного из показателей свежести используется несколько традиционных методов, включая высокоэффективную жидкостную хроматографию (ВЭЖХ) [36-38], капиллярный электрофорез (СЕ) [39], чрезвычайно эффективную жидкостную хроматографию с масс-спектрометрией (УВЕЙХ-МС) [40, 41], газовую хроматографию с масс-спектрометрией, (ГХ-МС) [42], масс-спектрометрию [43] и спектрофотометрические методы [44]. Несмотря на высокую селектив- ность и низкие пределы обнаружения, вышеупомянутые методы требуют очень дорогого оборудования, чрезвычайно трудоемки, требуют участия человека и являются экономически неэффективными [9, 45].
Нанотехнологии набирают популярность в пищевой промышленности, поскольку датчики на основе нанотехнологий успешно используются для измерения метаболитов пищевых продуктов [46 - 48]. Разработано несколько методов для анализа и мониторинга присутствия производных пурина в образцах мяса. Создание практических биосенсоров, позволяющих недорого и быстро обнаруживать низкие концентрации анализируемых веществ является предметом интенсивных исследований для проведения измерений в таких областях, как клиническая диагностика, мониторинг окружающей среды и безопасность пищевых продуктов. Успех биосенсоров, в отличие от традиционных аналитических методов, зависит от достижения надежности и точности для анализа в полевых условиях. Ключевым требованием является разработка оптимальной чувствительной поверхности, которая может стабилизировать биологические молекулы и быть сопряжена с физическими преобразователями, преобразующими данные биологического распознавания в сигнал, поддающийся количественной оценке [49 - 51].
Известны биосенсорные методы оценки свежести мяса на основе определения ксанти-ноксидазы. Широкое применение ксантиноксидазы для оценки свежести мяса обусловлено ее биосовместимостью, хорошими механическими свойствами, высокой активностью и стабильностью. Эти свойства, а также ее способность генерировать мощный и обратимый ток в присутствии кислорода делают ее пригодной для использования в биосенсорах [52]. Кроме того, ксантиноксидаза является ферментом, который катализирует окисление ксантина до мочевой кислоты и играет важную роль в метаболизме пуринов в организме [53]. Следовательно, ксан-тиноксидаза полезна для измерения концентрации ксантина и мочевой кислоты.
Авторами [54] предложен амперометрический ксантиновый биосенсор для определения свежести рыбного мяса. Сущность метода заключается в оценке количества присоединенных наночастиц ксантиноксидазы к золотым электродам (Au). Этот биосенсор показал предел обнаружения и линейность 0,01 мкм и 0,01–1,0 мкм соответственно. Использование наночастиц ксантиноксидазы в конструкции амперометрического ксантинового биосенсора упростило его изготовление, поскольку ксантиноксидазы были иммобилизованы посредством ковалентного связывания непосредственно с поликристаллическими электродами Au. Содержание гипоксантина в образцах рыбы также можно оценить с помощью разработанного биосенсора ксантиноксидазы и уриказы, иммобилизованного на поверхности пластины электрохимической полимеризацией паратолуолсульфоната полипиррола (PPy-pTS).
Несмотря на большие усилия и значительный прогресс в области биосенсоров, лишь немногие из них находят место на коммерческом рынке. Первым примером коммерческого биосенсора является ферментативный биосенсор глюкозы [42]. Ганбари и Неджабати [55] разработали многоамперометрический датчик на основе стеклоуглеродного электрода, модифицированного графеном / хитозаном / оксидом хрома (GCE / rGO / CS / Cr 2O3), для контроля уровней дофамина (УД), мочевой кислоты (АМ), ксантина (КС) и гипоксантина (ГК) в мышечной ткани. Датчик показал хорошую чувствительность к УД, Xa и ГК с постоянным линейным диапазоном 5–160, 10–500, 10–400 и 2–300 мкм. Язданпараст и др. [56] иммобилизовали ксан-тиноксидазу на стеклоуглеродном электроде, состоящем из многостенного нанокомпозита из углеродных нанотрубок и пленки из L-аспарагиновой кислоты. Демонстрация биосенсора была успешно проведена с помощью анализа ксантина в мышечной ткани рыбы с пределом обнаружения всего 3,5 × 10–4 мкм и линейным диапазоном 0,001–0,004 и 0,005-50,0 мкм. Сонг и др. [17] разработали новый биосенсор на основе ZnIn2S4 / UiO-66-NH, 2-модифицирован-ного стеклоуглеродного электрода для определения содержания ксантина и гипоксантина при оценке свежести рыбы. Включение UiO-66-NH2 улучшило функциональность биосенсора и обеспечило хорошее одновременное обнаружение ксантина и гипоксантина в широких линейных диапазонах 0,025–40 мкм и 0,3–40 мкм, а также низкие значения LOD 0,0083 мкм и 0,1 мкм.
Одно из основных направлений применения иерархических наноструктур заключается в разработке биосенсоров на основе полевых транзисторов для обнаружения различных анализируемых веществ. Биосенсор способен также специфически отличать комплементарную ДНК от ДНК с несовпадением одного основания, ДНК с несовпадением трех оснований и некомплементарной ДНК, что позволяет использовать его для оценки и скрининга однонуклеотидного полиморфизма. Высокочувствительный (2,87 × 105 А/А для 10 мМ глюкозы при V G = 20 В) и многоразовый биосенсор из диселенида вольфрама (WSe 2 ) был модифицирован GOx и получен биосенсор на основе глюкозы. Механически отшелушенные частицы WSe 2 обрабатывали плазмой слабой мощности O 2 для стимулирования химической активности перед иммобилизацией глюкозооксидазы посредством сшивания глутаровым альдегидом.
Пьерини и др. [31] был предложен простой, быстрый и недорогой метод определения содержания гипоксантина, ксантина и мочевой кислоты в образцах мяса с использованием электрода из пиролитического графита с плоской кромкой. Метод имел линейные диапазоны от 0,1 до 50 мкм для гипоксантина и ксантина и от 0,1 до 25,0 мкм для мочевой кислоты с LOD 0,08, 0,06 и 0,03 мкм соответственно.
Оптические датчики привлекли внимание исследователей к аналитическим приложениям, таким как мониторинг и оценка биологических и химических веществ. Оптические волокна обладают превосходной устойчивостью к помехам из внешней среды, что позволяет им быть высоконадежными и стабильными и в определенной степени расширяет область их применения. Для достижения сенсорной функции и повышения чувствительности часто используются специальные процедуры обработки для изменения геометрии волокна, чтобы нарушить первоначальный режим передачи с полным отражением. Методы обработки включают вытягивание конуса, сращивание сердцевины со смещением, лазерное травление и боковую шлифовку и полировку.
Лю с соавторами [57] использовали новый, простой, чувствительный и надежный флуоресцентный датчик на основе нуклеазы S1, окДНК, называемой FAM (ДНК-F), и оксида графена (GO) для определения свежести мяса КРС в присутствии аденозинтрифосфата (АТФ). В оптимальных условиях была достигнута линейная корреляция между флуоресценцией и концентрацией АТФ в диапазоне от 20 мкм до 3500 мкм с пределом обнаружения 3,2 мкм. Чен и соавторы [58] разработали флуоресцентный биосенсор с использованием наночастиц платины (Pt NP), которые обладают активностью, имитирующей пероксидазу, для быстрого обнаружения гипоксантина. Была показана линейная зависимость между интенсивностью флуоресценции и концентрацией гиоксантина в образцах со значениями от 8 до 2500 мкм. Предел обнаружения флуоресцентного биосенсора Pt-NPS составлял всего 2,88 мкм при отличной скорости извлечения 103,94–109,00 %.
Чжаном и соавторами [4] разработан метод флуоресценции, который показал предел обнаружения гиоксантина в образцах мяса от 0,7 ммоль.
Мултонгчун и Типа [59] разработали быстрый, чувствительный и экономичный колориметрический биосенсор (лабораторный на бумаге) для ферментативных каталитических реакций (ксантиноксидаза), который позволяет обнаруживать гипоксантин в образцах свежего и обработанного мяса в течение 5 мин. Метод основан на спектрофотометрическом определении гипоксантин и показал высокую точность, а также тот факт, что она не требует специальных инструментов, что является альтернативой традиционным методам. Линейный диапазон содержания гипоксантина составляет от 5 до 40 мг/л с пределом обнаружения 1,8 мг/л. Низкий предел обнаружения и широкий линейный диапазон лабораторного колориметра на бумаге указывают на высокую чувствительность и хорошую точность обнаружения и измерения концентрации гипоксантина в образцах свежего и обработанного мяса. Что касается аналитических свойств, все три ранее описанных колориметра обладают хорошей чувствительностью, точностью и безошибочностью при обнаружении и измерении концентраций ксантина или гипоксантина в различных типах образцов.
Ван и соавторы [60] разработали метод определения свежести мяса на основе определения присутствия гипоксантина (Hx) пероксии дазоподобной активности легированного кобальтом нитрида углерода графита (совместно допированный g-C3N4). Согласно исследованиям, Hx может быть обнаружен непосредственно спектральным поглощением на длине волны 652 нм с пределом обнаружения (LOD) 1,84 мг / кг и линейным диапазоном от 2,50 до 153,1 мг/кг. Низкое значение LOD и широкий линейный диапазон колориметра с сопутствующим легированием g-C3N4 указывают на высокую чувствительность и точность определения концентрации Hx в продуктах.
Мустафа и Андрееску [61] синтезировали надежное колориметрическое устройство без использования реагентов для контроля свежести мяса и прогнозирования порчи путем измерения уровня гипоксантина. Во время исследования оксид ксантина za и нитрозиния тетразо-лия хлорид (NBT) обрабатывали золь-гелем с биогидратом, в результате чего содержание гипоксантина в нем было низким – 3,7 мкм. Низкое значение LOD на колориметрическом приборе, не реагирующем на реакцию, указывает на высокую чувствительность при обнаружении гипоксантина в образцах мяса. Мустафа и др. [62] создали нанокатализатор, имитирующий фермент, со множеством функций в качестве имитатора пероксидазы, хромогенного индикатора и усилителя окислительно-восстановительных процессов, наночастиц церия (CENP) и биосенсора на основе ксантиноксидазы (XOD) для мониторинга и измерения уровня гипоксантина. CENP и XOD были иммобилизованы на силанизированной бумаге и показали LOD размером 15 мкм с линейным диапазоном до 800 мкм. Низкие значения LOD и широкий линейный диапазон колориметров CeNPs и XOD указывают на высокую чувствительность и точность определения концентрации гипоксантина.
Го и соавторы [45] разработали колориметрический метод определения гипоксантина (Hx) в мясе, основанный на пероксидазной активности суспензии ксантиноксидазы класса I (XOD-ASA) в сульфате аммония. Этот метод обладает хорошей избирательностью, низкой стоимостью и прост в приготовлении. Показано, что LOD составляет 6,93 мкм, и методика показала хорошую линейную зависимость в диапазоне от 20 до 200 мкм. В присутствии H 2 O 2 цвет меняется с бесцветного на синий. Низкое значение LOD и широкий линейный диапазон колориметра XOD-ASS демонстрируют высокую чувствительность и точность определения концентрации Hx в мясе. Дин и соавторы [63] разработали портативные гидрогели берлинской лазури с серебряным покрытием (SPB NPs) в сочетании с агарозными гидрогелями для обнаружения триметиламина (ТМА) в креветках и рыбе. Показано, что линейный диапазон составляет от 0,21 до 0,54 промилле. Использование смартфона и ручной тепловизионной камеры значительно повышает мобильность и удобство наблюдения на месте. Узкий линейный диапазон колориметра SPB NPs указывает на высокую точность определения и измерения концентрации ТМА в креветках и рыбе. Нереактивный колориметр имеет низкий LOD и не имеет определенного линейного диапазона, что указывает на высокую чувствительность при обнаружении гипоксантина в образцах мяса.
В таблице 1 показаны тенденции в области оптических биосенсоров для определения свежести мяса и его аналитических свойств в пищевой промышленности.
Таблица 1
Последние тенденции в области оптических биосенсоров для определения свежести мяса и их аналитических характеристик в пищевой промышленности [61 – 66]
|
Датчик |
Метод обнаружения |
Наноматериалы |
Образец |
Анализируемый |
|
1 |
2 |
3 |
4 |
5 |
|
Флуоресцентный-TPE-HPro/XO |
колориметрический |
- |
рыба, мясо |
Hx |
|
O-CDS |
колориметрический |
углеродные точки |
рыба |
Hx |
|
XOD-ASS |
флуоресценция |
- |
рыба, мясо |
Hx |
Продолжение таблицы 1
|
1 |
2 |
3 |
4 |
5 |
|
Флуоресцентные-PtNPs |
флуоресценция |
наночастицы платины |
рыба, креветки, кальмары |
Hx |
|
Флуоресцентный-NH 2 - Cu-MOF нанолист |
флуоресценция |
нанолистовые металлоорганические каркасы (MOF) |
рыба, мясо |
Hx |
|
ДНК-F / GO |
флуоресценция |
оксид графена (GO) |
говядина |
ATP |
|
Cys-CuNCs |
флуоресценция |
нанокластеры меди |
рыба |
Hx |
|
Наноматериалы XOD@ZnO |
колориметрический |
наноматериалы из оксида цинка (ZnO) |
рыба |
Xa |
|
Ионы серебра и β-D-GP |
волоконно-оптический |
- |
рыба, мясо |
BAs |
|
AgP |
колориметрический |
нанопластинки серебра (AgP) |
рыба, мясо |
Xa |
|
Бумажный колориметрический биосенсор |
флуоресценция |
- |
говядина, свина, рыба |
Hx |
|
Совместное добавление g-C 3 N 4 |
колориметрический |
- |
рыба |
Hx |
|
XO / NBT / золь-гель биогибрид |
колориметрический |
- |
рыба тилапия |
Hx |
|
CeNPs / XOD /silanized paper |
колориметрический |
наночастицы оксида церия (CENP) |
разложившаяся рыба |
Hx |
|
SPB NPS / агарозный гидрогель |
флуоресценция/коло-риметрия |
наночастицы берлинской лазури, легированные серебром (SPB NPs) |
креветки, рыба |
TMA |
|
Колориметрические наностержни CTAB-Au |
флуоресценция |
золотые наностержни (GNR) |
рыба, мясо |
Hx |
В целом биосенсоры, основанные на нанотехнологиях, как правило, имеют более низкие показатели LOD по сравнению с традиционными методами. Например, содержание ксантина в мясе рыбы, по данным амперометрии (XODNPs/Au), составляет 0,01 мкм, в то время как содержание ксантина, по данным ВЭЖХ-УФ, составляет 0,0774 мг/л. Аналогично содержание гипоксантина в рыбе составляет 3,93 мкм при использовании наночастиц NH-флуоресценции 2 Cu MOF, тогда как содержание гипоксантина составляет 0,0555 мг / л при использовании ВЭЖХ-УФ (на основе табл. 2). Этот более низкий LOD может сделать биосенсоры на основе нанотехнологий более подходящими для определения следовых концентраций анализируемых веществ.
В таблице 2 показаны аналитические свойства традиционных методов и биосенсоров на основе нанотехнологий.
Таблица 2
Сравнение аналитических характеристик традиционных методов и биосенсоров на основе нанотехнологий
|
Метод анализа |
Образец |
Анализируемый |
Линейный диапазон |
LOD-предел обнаружения |
Ссылка |
|
1 |
2 |
3 |
4 |
5 |
6 |
|
Традиционные |
|||||
|
ВЭЖХ-DAD Высокоэффективная жидкостная хроматография |
коровье молоко |
аллантоин, мочевая кислота, Xa, Hx |
3,125–100 мкг/мл |
0,74 мкг/мл 0,16 мкг/мл 0,09 мкг/мл 0,14 мкг/мл |
[10] |
Продолжение таблицы 2
|
1 |
2 |
3 |
4 |
5 |
6 |
|
Au-PEDOT-fMWCNT/GCE |
мясо рыбы |
UA Xa Hx |
0,1–800 мкм 0,05–175 мкм 0,1–150 мкм |
199,3 нМ 24,1 нМ 90,5 нМ |
[14] |
|
ВЭЖХ-УФ |
морская рыба |
аденин, гуанин, Hx, Xa |
0,1–300 мг/л |
0,0774 мг/л 0,0178 мг/л 0,0118 мг/л 0,0555 мг/л |
[33] |
|
ВЭЖХ-УФ |
сырые анчоусы |
аденин, гуанин, Hx, Xa |
- |
- |
[37] |
|
ВЭЖХ-УФ |
рыба, моллюски |
аденин, гуанин, Hx, Xa |
0,05–300 мг/л |
0,02 мг/л 0,03 мг/л 0,06 мг/л 0,10 мг/л |
[39] |
|
UHPLC-MS |
сыворотка |
23 производных пурина |
0,002–11,2 мкг/мл |
0,05–6,3 нг/мл |
[41] |
|
ВЭЖХ-VWD |
куриный бульон по-китайски |
аденин, гуанин, Hx, Xa, мочевая кислота |
0,05–100 мг/л |
0,66 мкг/л 0,64 мкг/л 0,58 мкг/л 1,14 мкг/л 1,71 мкг/л |
[63] |
|
Биосенсор на основе нанотехнологий |
|||||
|
Амперометрия (XODNPs/Au) |
мясо, рыба |
Xa |
0,01–1,0 мкм |
0,01 мкм |
[54] |
|
Электрод XO/Poly (l- Asp)/MWCNT/G CE |
мясо, рыба |
Xa |
0,001–0,004 мкм |
3,5 × 10-4 мкм |
[56] |
|
Флуоресцент-ный-NH 2 -Cu-MOF нанолист |
рыба |
Hx |
10 - 2000 мкм |
3,93 мкм |
[64] |
|
CeNPs/XOD /silanized paper |
разложившаяся рыба |
Hx |
800 мкм |
15 мкм |
[62] |
Примечание. Нх – гипоксантин; Ха – ксантин; UA – мочевая кислота.
Традиционные методы, как и биосенсоры на основе нанотехнологий, обладают хорошей чувствительностью и точностью при обнаружении в образцах мяса различных анализируемых веществ, но существуют некоторые различия в их аналитических свойствах. Биосенсоры на основе нанотехнологий, как правило, имеют более широкий линейный диапазон и более низкие пределы обнаружения по сравнению с традиционными методами. Это может способствовать определению более широкого диапазона концентраций анализируемых веществ или компонентов. Однако важно отметить, что выбор метода в конечном счете зависит от конкретных требований и ограничений области применения, и оба типа методов имеют свои преимущества и ограничения.
Заключение
Определение свежести пищевых продуктов, в том числе мяса и мясопродуктов, остается актуальным направлением научных исследований пищевой отрасли. Существуют общепринятые методы исследований на основании органолептических, физико-химических и микробиологических показателей. Находят определенное применение в идентификации веществ - критериев свежести пищевой продукции - инструментальные методы. Особенно развиваются новые методы по определению свежести мяса на основе биосенсоров и протеомики.
Список литературы Современные методы определения свежести мяса: мини-обзор
- Бурханова А.Г., Баженова Б.А., Егорова Р.А. и др. Исследование хранимоспособности рубленных полуфабрикатов с введением лука угловатого Allium Angulosum L. // Вестник ВСГУТУ. - 2022. - № 1 (84). - С. 5-14.
- Егорова Р.А., Баженова Б.А., Бурханова А.Г. и др. Влияние лука угловатого Allium Angulosum L. на процесс окисления разных видов животного жира при хранении // Вестник ВСГУТУ. - 2020. - № 1 (76). - С. 26-36.
- Chen J., Kong Q., Sun Z. et al. Freshness analysis based on lipidomics for farmed Atlantic salmon (Salmo salar L.) stored at different times // Food Chem. - 2022. - N 373. - Р. 131564.
- Zhang Z., Kwok R.T., Yu Y. et al. Aggregation-induced emission luminogen-based fluorescence detection of hypoxanthine: A probe for biomedical diagnosis of energy metabolism-related conditions // J. Mater. Chem. B. - 2018. - N 6. - P. 4575-4578.
- Guo X., Wang X., Huang D. et al. Method study on determination of total purine content in fish meat by diazotization reaction combined with SERS // LWT. - 2020. - N 123. - P. 109027.
- Jia W., Fan Z., Shi Q. et al. LC-MS-based metabolomics reveals metabolite dynamic changes during irradiation of goat meat. Int. // Food Res. J. - 2021. - N 150. - P. 110721.
- Daldal Y.D., Demiralay E.Q. Chromatographic and UV-visible spectro-photometric pKa determination of some purine antimetabolites // J. Mol. Liq. - 2020. - N 317. - P. 113930.
- Mathew M.R., Kumar K.G. Poly Amino Hydroxy Naphthalene Sul-phonic Acid) Modified Glassy Carbon Electrode // An Effective Sensing Platform for the Simultaneous Determination of Xanthine and Hypoxanthine // J. Electrochem. Soc. - 2020. - N 167. - P. 047519.
- Dervisevic M., Dervisevic E., Senel M. Recent progress in nanomateri-al-based electrochemical and optical sensors for hypoxanthine and xanthine // A review. Mikrochim. Acta. - 2019. - N 186. - P. 749.
- Vlassa M., Filip M., Dragomir C. Simultaneous quantifications of four purine derivatives bi-omarkers in cow milk by SPEH PLC-DAD // Czech J. Food Sci. - 2021. - N 39. - P. 122-130.
- Nanda P.K., Bhattacharya D., Das J.K. et al. Emerging Role of Biosensors and Chemical Indicators to Monitor the Quality and Safety of Meat and Meat Products // Chemosens. - 2022. - N 10. - P. 322.
- Thakur D., Pandey C.M., Kumar D. Highly Sensitive Enzymatic Bio-sensor Based on Polyaniline-Wrapped Titanium Dioxide Nanohybrid for Fish Freshness Detection // Appl. Biochem. Biotechnol. - 2022. - N 194. - P. 3765-3778.
- Tripathi A., Elias A.L., Jemere A.B. et al.. Amperometric De-termination of Xanthine Using Nanostructured NiO Electrodes Loaded with Xanthine Oxidase // ACS Food Sci. Technol. - 2022. - N 2. -P.1307-1317.
- Sen S., Sarkar P. A simple electrochemical approach to fabricate func-tionalized MWCNT-nanogold decorated PEDOT nanohybrid for simultaneous quantification of uric acid, xanthine and hypoxanthine // Anal. Chim. Acta. - 2020. - N 1114. - N 15-28.
- Wang G., Sun J., Yao Y. et al. Detection of Inosine Monophosphate (IMP) in meat using doubleenzyme sensor // Food Anal. Methods. - 2020. - N 13. - P. 420-432.
- Zhang Y., Gao X., Ye Y. et al. Fe-Doped polydopamine nanoparti-cles with peroxidase-mimicking activity for the detection of hypoxanthine related to meat freshness // Analyst. - 2022. - N 147. - P. 956-964.
- Song D., Chen Q., Zhai C. et al. Label-Free ZnIn2S4/UiO-66-NH2 Modified Glassy Carbon Electrode for Electrochemically Assessing Fish Freshness by Monitoring Xanthine and Hypoxanthine // Chemosens. - 2022. - N 10. - P. 158.
- Liu R., Warner R.D., Zhou G. et al. Contribution of nitric oxide and protein S-nitrosylation to variation in fresh meat quality // Meat Sci. - 2018, - N144. - P. 135-148.
- Cenci-Goga B.T., IuliettoM.F., Sechi P. et al. New trends in meat packaging // Microbiol. Res. -2020. N 11. - P. 56-67.
- Mohammed H.H.H., Jin G., Ma M. et al. Comparative characterization of proximate nutritional compositions, microbial quality and safety of camel meat in relation to mutton, beef, and chicken // LWT. -2020. - N 118. - P. 108714.
- Johnson J., Atkin D., Lee K. et al. Determining meat freshness using electrochemistry: Are we ready for the fast and furious? // Meat Sci. - 2019. - N 150. - P. 40-46.
- Alvarez S., MullennA.M., Hamill R. et al. Dry-aging of beef as a tool to improve meat quality. Impact of processing conditions on the technical and organoleptic meat properties // Adv. Food Nutr. Res. -2021. - N 95. - P. 97-130.
- Rey A.I., Menoyo D., Segura J. et al. Combina-tion of dietary glycaemic index and fasting time prior to slaughter as strategy to modify quality of pork // Meat Sci. - 2020. - N 161. - P. 108013.
- Chauhan S.S., England E.M. Postmortem glycolysis and glycogenolysis: Insights from species comparisons // Meat Sci. - 2018. - N 144. - P. 118-126.
- Lin W.C., He Y.M., Shi C. et al. ATP catabolism and bacterial succession in postmortem tissues of mud crab (Scylla paramamosain) and their roles in freshness // Int. Food Res. J. - 2022. - N 155. - P. 110992.
- Rongsheng Z., Huaizhong W., Song L. et al. Deposition Pattern of Inosine Monophosphate (IMP) in Pig Muscle during Cold Storage // Anim. Feed Sci. - 2017. - N 9. - P. 197-218.
- Feng X., Moon, S.H., Lee H.Y. et al. Effect of irradiation on the degradation of nucleotides in turkey meat // LWT. - 2016. - N 73. - P. 88-94.
- Hong H., Regenstein J.M., Luo Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev // Food Sci. Nutr. - 2017. - N 57. - P. 1787-1798.
- Ebata K., Yamashita Y., Inohara K. et al. Evaluation of Muscle Post-mortem Changes of Japanese Anchovy Engraulis japonicus and Round Herring Etrumeus teres and Recovery of ATP Concentration of Japanese Anchovy Following Brief Rest in a Fish Cage // J. Fish. Eng. - 2020. - N 56. - P. 149-158.
- Huang Z., Zhang J., Gu Y. et al. Research progress on inosine monophosphate deposition mechanism in chicken muscle // Crit. Rev. Food Sci. Nutr. - 2022. - N 62. - P. 1062-1078.
- Pierini G.D., Robledo S.N., Zon M.A. et al. Development of an electroanalytical method to control quality in fish samples based on an edge plane pyrolytic graphite electrode. Simultaneous determination of hypoxanthine, xanthine and uric acid // Microchem. J. - 2018. - N 138. - P. 58-64.
- Karim N.U., Kennedy J.T., Linton M. et al. Determination of nucleotide and enzyme degradation in haddock (Melanogrammus aeglefinus) and herring (Clupea harengus) after high pressure processing // Peer J. - 2019. - N 7. - P. 7527.
- Li J., Zhou G., Xue P. et al. Spoilage microbes' effect on freshness and IMP degradation in sturgeon fillets during chilled storage // Food Biosci. - 2021. - N 41. - P. 101008.
- Min J.G., Joung B.C., Jung W.Y. Postmortem Changes in Spinal Cord-damaged Olive Flounder (Paralichthys olivaceus) // J. Food Nutr. Res. - 2019. - N 7. - P. 500-505.
- Yoshioka T., Konno Y., Konno K. Below-zero storage of fish to suppress loss of freshness // Fish. Sci. - 2018. - N 85. - P. 601-609.
- Felicia W. X. L., Rovina K., Nur'Aqilah N. et al. Assessing Meat Freshness via Nanotechnology Biosensors: Is the World Prepared for Lightning-Fast Pace Methods? // Biosensors. - 2023. - N 13. - P. 217.
- Takayanagi F., Fukuuchi T., Yamaoka N. et al. Measurement of the total purine contents and free nucleosides, nucleotides, and purine bases composition in Japanese anchovies (Engraulis japonicus) using highperformance liquid chromatography with UV detection // Nucleos. Nucleat. Nucl. - 2020. - N 39. - P. 1458-1464.
- Qu X., Sui J., Mi N. et al. Determination of four different purines and their content change in seafood by high-performance liquid chromatography // J. Sci. Food Agric. - 2017. - N 97. - P. 520-525.
- Felisiak K., SzymczakM., Kotakowski E. Identification of non-protein nitrogen compounds separated by CZE without derivatization from TCA extract from salted herring meat // J. Food Compos. Anal. -2019. - N 77. - P. 108-114.
- Ali N.S.M., Zabidi A.R., Manap M.N.A. et al. Effect of different slaughtering methods on metabolites of broiler chickens using Ultra High-Performance Liquid Chromatography-Time of Flight-Mass Spectrometry (UHPLC-TOF-MS) // Food Res. - 2020. - N 4. - P. 133-138.
- Zheng Y., LiX., Chen X. et al. Simultaneous determination of amino acids, purines and derivatives in serum by ultrahigh-performance liquid chromatography/tandem mass spectrometry // RCM. - 2019. - N 33. - P.81-88.
- Ueda S., Yamanoue M., Sirai Y. et al. Exploring the Characteristic Aroma of Beef from Japanese Black Cattle (Japanese Wagyu) via Sensory Evaluation and Gas Chromatography-Olfactometry // Metabolites. - 2021. - N 11. - P. 56.
- Chang W.C. W., Wu H.Y., Yeh Y.et al. Untargeted foodomics strategy using high-resolution mass spectrometry reveals potential indicators for fish freshness // Anal. Chim. Acta. - 2020. - N 1127. - P. 98-105.
- Dibirasulaev M., Belozerov G., Arkhipov L. et al. Quick and simple spectrophotometry method of identification of the thermal state of meat on the basis of composition and content of free nucleotides // Food Sci. Nutr. - 2018. - N 9. - P. 572-583.
- Guo C., You S., Li C. et al. One-Step and Colorimetric Detection of Fish Freshness Indicator Hypoxanthine Based on the Peroxidase Activity of Xanthine Oxidase Grade I Ammonium Sulfate Suspension. Front // Microbiol. - 2021 - N 12. - P. 791227.
- Prajapati S., Padhan B., Amulyasai B. et al. Nanotechnology-based sensors. Biopolym // Based Formul. - 2020. - N 1. - P. 237-262.
- Bashir O., Bhat S.A., Basharat A. et al. Nano-engineered materials for sensing food pollutants: Technological advancements and safety issues // Chemosphere. - 2022. - N 292. - P. 133320.
- Erna K.H., Felicia W.X.L., Rovina K. et al. Development of curcumin/rice starch films for sensitive detection of hypoxanthine in chicken and fish meat // Carbohydr. Polym. - 2022. - N 3. - P. 100189.
- Bonnet C., Bouamra-Mechemache Z., Réquillart V. et al. Regulating meat consumption to improve health, the environment and animal welfare // Food Policy. - 2020. - N 97. - P. 101847.
- Erna K.H., Rovina K., Mantihal S. Current. Detection Techniques for Monitoring the Freshness of Meat-Based Products: A Review // J. Package. Technol. Res. - 2021. - N 5. - P. 127-141.
- Albelda J.A., Uzunoglu A., Santos G.N.C. et al. Graphene-titanium dioxide nanocomposite based hypoxanthine sensor for assessment of meat freshness // Biosens. Bioelectron. - 2017. - N 89. - P. 518-524.
- Boluda A., Casado C.M., Alonso B. et al. Efficient Oxidase Biosensors Based on Bioelectrocata-lytic Surfaces of Electrodeposited Ferrocenyl Polycyclosiloxanes - Pt Nanoparticles // Chemosensors. - 2021. - N 9. - P. 81.
- Furuhashi M. New insights into purine metabolism in metabolic diseases: Role of xanthine oxi-doreductase activity // Am. J. Physiol. Endocrinol. Metab. - 2020, - N 319, E827-E834.
- Joon A., Ahlawat J., Aggarwal V. et al. An improved amperometric determination of xanthine with xanthine oxidase nanoparticles for testing of fish meat freshness // Sens. Bio-Sens. Res. - 2021. - N 33. -P.100437.
- Ghanbari K., Nejabati F. Ternary nanocomposite-based reduced graphene oide/chitosan/Cr2O3 for the simultaneous determination of dopamine, uric acid, xanthine, and hypoxanthine in fish meat // Anal. Methods. - 2020. - N 12. - P. 1650-1661.
- Yazdanparast S., Benvidi A., Abbasi S. et al. Enzyme-based ultrasensitive electrochemical biosensor using poly (l-aspartic acid)/MWCNT bio-nanocomposite for xanthine detection: A meat freshness marker // Microchem. J. - 2019. - N 149. - P. 104000.
- Liu Z., Zhong Y., Hu Y. et al. Fluorescence strategy for sensitive detection of adenosine triphos-phate in terms of evaluating meat freshness // Food Chem. - 2019. - N 270. - P. 573-578.
- Chen J., Lu Y., Yan F. et al. A fluorescent biosensor based on catalytic activity of platinum nanoparticles for freshness evaluation of aquatic products // Food Chem. - 2020. - N 310. - P. 125922.
- Mooltongchun M., Teepoo S. A simple and cost-effective microfluidic paper-based biosensor analytical device and its application for hypoxanthine detection in meat samples // Food Anal. Methods. - 2019 -N 12. - P. 2690-2698.
- Wang X., Lin Z.Z., Hong C.Y. et al. Colorimetric detection of hypoxanthine in aquatic products based on the enzyme mimic of cobalt-doped carbon nitride // New J. Chem. - 2021. - N 45. - P. 18307-18314.
- Mustafa F., Andreescu S. Based enzyme biosensor for one-step detection of hypoxanthine in fresh and degraded fish // ACS Sens. - 2020. - N 5. - P. 4092-4100.
- Mustafa F., Othman A., Andreescu S. Cerium oxide-based hypoxanthine biosensor for Fish spoilage monitoring // Sens. Actuators B Chem. - 2021. - N 332. - P. 129435.
- Ding N., Dong S., Zhang Y. et al. Portable silver-doped prussian blue nanoparticle hydrogels for colorimetric and photothermal monitoring of shrimp and fish freshness // Sens. Actuators B Chem. - 2022. -N 363. - P. 131811.
- Garg D., Singh M., Verma N. Review on recent advances in fabrication of enzymatic and chemical sensors for hypoxanthine // Food Chem. - 2021. - N 375. - P. 131839.
- Mu G., Luan F., Xu L. et al. Determination of purines in soybean milk by capillary electrophoresis in comparison with high performance liquid chromatography // Anal. Methods. - 2012. - N 4. - P. 3386-3391.
- Hu S., Yan J., HuangX. et al. A sensing platform for hypoxanthine detection based on aminofunc-tionalized metal organic framework nanosheet with peroxidase mimic and fluorescence properties // Sens. Actuators B Chem. - 2018. - N 267. - P. 312-319.


