Современные возможности безопиоидного послеоперационного обезболивания
Автор: Ефременко Ирина Валерьевна, Овечкин Алексей Михайлович
Журнал: Хирургическая практика @spractice
Рубрика: Обзоры литературы
Статья в выпуске: 1, 2013 года.
Бесплатный доступ
Состояние послеоперационного обезболивания продолжает оставаться неудовлетворительным во всем мире. Возможности применения опиоидных анальгетиков во многом исчерпаны, сопряжены с рядом осложнений, кроме того, жестко регламентированы законами Российской Федерации. Препараты группы НПВС имеют серьезные противопоказаний со стороны ряда пациентов. В связи с этим, особый интерес представляют средства, воздействующие на ^метил-Б-аспартат ионные рецепторы, играющие важную роль в механизмах формирования острой боли. В данном обзоре рассматривается эффективность применения микродоз кетамина, антиковульсанта габапентина, а также сульфата магния в схемах послеоперационного обезболивания.
Послеоперационное обезболивание, nmda-рецепторы, вторичная гиперальгезия, кетамин, габапентин, сульфат магния
Короткий адрес: https://sciup.org/142211501
IDR: 142211501
Текст обзорной статьи Современные возможности безопиоидного послеоперационного обезболивания
Состояние проблемы
Проблема адекватности послеоперационного обезболивания с годами, к сожалению, не теряет своей актуальности. В конце прошлого века около 50% больных хирургического профиля оценивали послеоперационное обезболивание как неадекватное. По данным Национального центра статистики здравоохранения США, от острой послеоперационной боли ежегодно страдает более 4,3 млн американцев, 50% из них считают послеоперационное обезболивание неадекватным [25]. В другом исследовании указано, что в 1-е и даже 2-е сутки после операции боль характеризуется средней и высокой интенсивностью у 80% пациентов [1]. В одном из наиболее крупных исследований (около 20 тыс. пациентов хирургических отделений Великобритании) послеоперационные болевые ощущения средней интенсивности были отмечены в 29,7% (26,4–33%) случаев, высокой интенсивности – в 10,9% (8,4–13,4%) случаев [9]. Анализ качества послеоперационного обезболивания в Германии (25 клиник, 2252 пациента) показал, что боль средней и высокой интенсивности в покое испытывали 29,5% пациентов, а при активации – более 50%, при этом 55% всех пациентов были не удовлетворены качеством обезболивания [19] Не так давно проведенное в Европе масштабное эпидемиологическое исследование PATHOS, включившее 7 стран центральной и южной Европы (746 клиник), в очередной раз выявило неудовлетворительное качество послеоперационного обезболивания, и необходимость принятия неотложных мер по его улучшению [3].
Опиоидная анальгезия и ее недостатки
Опиоидная анальгезия (в частности, внутривенное введение морфина в режиме «анальгезии, контролируемой пациентом», т.е. аутоанальгезии) продолжает оставаться «золотым стандартом» послеоперационного обезболивания в большинстве европейских клиник. Однако, по мнению ряда зарубежных специалистов, послеоперационное назначение опиоидных анальгетиков ассоциируется с увеличением числа осложнений послеоперационного периода, а также увеличивает стоимость пребывания пациента в клинике [22, 38]. Помимо всем известных побочных эффектов препаратов данного ряда (угнетение дыхания, тошнота и рвота, угнетение перистальтики ЖКТ, спазм сфинктера Одди, кожный зуд), сегодня рассматриваются и такие нежелательные эффекты опиоидов, как иммуносупрессия (особенно для морфина), а также индуцированная опиоидами гиперальгезия (снижение порогов активации ноцицепто-ров) и формирование острой толератности к опиоидам.
Вообще, гипералгезии как индуцированной самим хирургических вмешательством, так и обусловленной введением некоторых препаратов, в настоящее время уделяется огромное внимание в борьбе с послеоперационной болью.
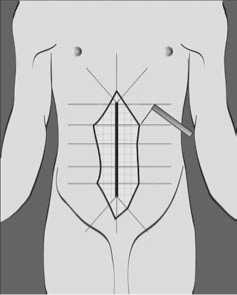
Известнейший специалист в области патофизиологии периоперационного периода, датский хирург Хенрик Келет неоднократно высказывал идеи о необходимости разработки безо-пиоидных или практически безопиоидных методик послеоперационного обезболивания, использование которые способствует ранней послеоперационной реаби-
Рис. 1. Схематичное изображение литации пациентов [15, 16]. зоны вторичной гиперальгезии вокруг
Первым шагом повы-операционной раны шения качества периопе-рационного обезболивания должно являться обобщение и внедрение в клиническую практику имеющихся данных доказательной медицины о целесообразности применения в схемах послеоперационного обезболивания препаратов, оказывающих воздействие на NMDA-рецепторный комплекс*. Степень активности NMDA-рецепторов играет ключевую роль в механизмах формирования гиперальгезии и острой боли в целом. В данном обзоре мы рассмотрим возможности использования в данном качестве кетамина, габапентина и сульфата магния. Ни один из этих препаратов не является анальгетиком как таковым, однако грамотное их применение позволяет решить многие проблемы послеоперационного обезболивания.
Кетамин
Препарат был синтезирован еще в 1962 году, однако свойства неконкурентного антагониста NMDA-рецепторов, способного препятствовать развитию гипералгезии, были выявлены у него лишь в 1990 году. Кетамин связывается с фенциклидиновыми рецепторами внутренней поверхности каналов NMDA- рецепторов. Таким образом, он препятствует формированию гипервозбудимости спинальных нейронов.
Продемонстрировано, что в/в инфузия кетамина в течение 3-х суток после операции уменьшает зону механической гипе-ральгезии вокруг операционной раны на протяжении 7 суток наблюдения у пациентов, перенесших нефрэктомию [27]. Т.е. эффект нельзя объяснить собственно анальгетическим действием препарата, а именно его превентивным влиянием в отношении формирования гиперальгезии.
В другом исследовании была установлена прямая зависимость между площадью зоны гиперальгезии в первые 72 часа после операций на толстом кишечнике и наличием, а также интенсивностью болевых ощущений спустя 1 месяц, полгода и 1 год [7]. Авторы делают вывод, что использование относительно больших доз опиоидных анальгетиков короткого действия без добавления малых доз кетамина, уменьшающего площадь зоны гиперальгезии, опасно формированием хронического послеоперационного болевого синдрома (ХПБС).
При анализе результатов 37 исследований (2385 пациентов), добавление малых доз кетамина к стандартной опиоидной анальгезии было признано целесообразным в 54% случаев [28]. При этом авторы подчеркнули, что при лапароскопических и артроскопических хирургических вмешательствах, тонзилэктомиях стандартное послеоперационное назначение комбинации НПВС + парацетамол + инфильтрация местными анестетиками позволяют успешно решить проблему послеоперационной боли. В этих ситуациях целесообразность добавления кетамина не очевидна. Авторы считают, что она выше после обширных травматичных операций, когда в послеоперационном периоде требуется назначение значительных доз опиоидных анальгетиков.
Исследовано влияние кетамина и продленной эпидуральной анальгезии на величину зоны гиперальгезии у пациентов, перенесших резекции толстого кишечника по поводу злокачественных новообразований [17]. Введение кетамина предусматривало первичный болюс 0,5 мг/кг с последующей инфузией со скоростью 0,25 мг/кг/час. В группах, где проводилась ЭА + кетамин, площадь гиперальгезии на всех этапах исследования не превышала 5–10 см2. Через 1 и 6 месяцев после операции в этой группе остаточные болевые ощущения сохранялись у 62 и 48% пациентов соответственно. Не имели никаких болевых ощущений пациенты, у которых ЭА была начата до разреза и продолжена в течение 72 часов послеоперационного периода.
В группе, получавшей кетамин, но не получавшей ЭА, через 24 часа после опера-ции зона гиперальгезии составляла около 10 см2, через 48 и 72 часа – 75 и 155 см2 соответственно. Авторы сделали вывод, что оптимальным является сочетание интра- и послеоперационной ЭА с в/в инфузией кетамина.
NMDA-рецепторный антагонист кетамин не только предупреждает развитие ранней гиперальгезии, но и усиливает анальгетический эффект опиоидов. В эксперименте было продемонстрировано, что предшествующее назначение фентанила снижает анальгетический потенциал назначенного после этого морфина. Превентивное (до фентанила) введение кетамина

усиливает анальгетический эффект фентанила и восстанавливает исходный анальгетический потенциал морфина [18].
Этот факт имеет существенное клиническое значение, поскольку неадекватность послеоперационного обезболивания в ряде случаев может быть обусловлена вариабельностью индивидуальной чувствительности к опиоидным анальгетикам. Насколько значимой является данная проблема? В исследовании, оценивавшем эффективность в/в болюсного введения морфина по 2 мг с интервалом 4–5 минут у пациентов, перенесшие плановые абдоминальные или торакальные вмешательства [37], морфин-резистентными оказались 145 из 1088 исследованных пациентов (22%). Недостаточную эффективность послеоперационной анальгезии морфином однозначно связывают с активацией NMDA-рецепторов [20]. NMDA-рецепторы активируются как самим по себе хирургическим вмешательством, так и вводимыми пациенту опиоидными анальгетиками. Блокада этих рецепторов до разреза позволяет предотвратить развитие вторичной гиперальгезии, снизить интенсивность боли и потребность в назначении анальгетиков у пациентов, в определенной степени резистентных к действию опиоидов.
В последние годы все более актуальной становится проблема проведения анестезии и послеоперационной анальгезии у наркозависимых пациентов. Толератность к опиоидам требует использования их в высоких дозах. Эффективность анальгезии при этом достигается далеко не всегда, более того, существует риск развития гиперальгезии и разнообразных побочных эффектов. В одно из исследований были включены пациенты, страдающие зависимостью к наркотикам и перенесшие операции заднего спондилодеза в условиях общей анестезии [35]. В основной группе пациенты получали 0,2 мг/кг кетамина во время индукции с последующей инфузией 2 мкг/кг/час до перевода из палаты послеоперационного наблюдения. В качестве основного анальгетика использовался гидроморфон в режиме КПА. Пациенты в группе кетамина имели достоверно меньшую интенсивность боли в 1-е сутки после операции. У 3 из 12 пациентов группы сравнения не удалось достичь сколь-нибудь приемлемого качества обезболивания при помощи опиоидных анальгетиков и пришлось тоже назначить кетамин.
Каким образом оптимизировать использование кетамина? Большое значение имеет способ назначения препарата. Наиболее эффективна внутривенная инфузия. По данным большинства исследований [2, 27, 29], наиболее полный превентивный эффект достигается при продленном назначении кетамина в течение нескольких суток (чаще 3-х). Высокая интенсивность тканевых повреждений и длительность послеоперационного болевого синдрома при операциях высокой травматичности (абдоминальная, торакальная хирургия), в большинстве случаев не позволяют однократной инъекции кетамина до разреза сколь-нибудь значимо влиять на интенсивность боли. Наиболее распространенная и эффективная схема назначения кетамина предусматривает в/в введение болюса 0,3–0,5 мг/кг в процессе индукции анестезии с последующей инфузией 3–5 мкг/ кг/мин до окончания операции и 1,5–2 мкг/кг/мин в течение 48–72 часов после нее.
Какой же вывод можно сделать на основании анализа вышеупомянутых многочисленных исследований? Заслуживает ли кетамин права быть включенным в схемы анестезии и послеоперационной анальгезии? Обратимся к данным доказательной медицины самого высокого уровня.
Систематизированный обзор исследований, оценивавших предупреждающее влияние кетамина на формирование послеоперационного болевого синдрома, был выполнен в 2004 году [21] на основании анализа публикаций, представленных в базах данных «MEDLINE» (1966–2003 гг.) и «EMBASE» (1985–2003 гг.). Критериям включения отвечали 24 исследования. В них были представлены все способы назначения кетамина. Используемые дозы варьировали от 0,15 до 1 мг/кг. В 14 из 24 (58%) работ было подтвержден превентивный эффект кетамина в отношении развития послеоперационного болевого синдрома, поскольку длительность эффекта существенно превышала длительность действия самого кетамина
Таким образом, сегодня можно сделать вывод, что кетамин обладает несомненным предупреждающим эффектом в отношении развития интенсивного послеоперационного болевого синдрома. Главным условием эффективности предупреждающей анальгезии должно являться предотвращение активации NMDA-рецепторов. Можно утверждать, что использование относительно больших доз опиоидных анальгетиков короткого действия во время операции без добавления малых доз кетамина опасно формированием ХПБС.
Габапентин
Габапентин был внедрен в клиническую практику в 1993 году в качестве противосудорожного препарата второго поколения. Вскоре была выявлена его эффективность в лечении ряда хронических нейрогенных болевых синдромов, в частности, постгерпетической невралгии, диабетической нейропатии, тригеминальной невралгии и т.д. В 2002 г. в журнале «Anesthesiology» были представлены данные, позволившие переосмыслить клиническое значение габапентина и рассматривать его как анальгетик широкого спектра действия [8].
Традиционно, патофизиологические механизмы и принципы лечения острой послеоперационной и хронической нейропатической боли рассматривались раздельно, однако на сегодняшний день выявлены некоторые общие патофизиологические механизмы, связывающие острые ноцицептивные и хронические нейропатические болевые синдромы. Установлено, что послеоперационная боль имеет как ноцицептивный, так и нейропатический компоненты [5, 26]. Ноцицептивный обусловлен активацией периферических ноцицепторов с формированием ноцицептивных стимулов и проведением их по афферентным волокнам в задние рога спинного мозга, а оттуда – в супраспинальные структуры. Местные анестетики, НПВС, опиоидные анальгетики позволяют достаточно успешно препятствовать реализации вышеуказанных механизмов. Нейропатический компонент связан с повреждением нервных волокон и характеризуется изменениями модуляции боли, а также центральной сенситизацией. Клиническим результатом является формирование гиперальгезии.
В 2004 году были проанализированы данные 7 исследований, посвященных эффективности назначения габапентина с целью послеоперационного обезболивания [6]. В 6 из них было выявлено достоверное снижение потребности пациентов в дополнительном назначении анальгетиков в течение 24 часов после операции. Пациенты, включенные в данные исследования, перенесли гистерэктомии, операции на позвоночнике, радикальные мастэктомии, лапароскопические холецистэктомии.
Каковы механизмы анальгетического действия препарата? Антиноцицептивный эффект обусловлен связыванием препарата с альфа2-дельта субъединицами пресинаптических потенциал-зависимых Са2+-каналов нейронов задних рогов спинного мозга, функциональная активность и количество которых резко увеличивается при повреждении периферических тканей [5]. Активация α2δ-субъединиц усиливает поток ионов Са2+ по кальциевым каналам и удлиняет деполяризацию [14]. Повышенный вход ионов Са2+ в клетку увеличивает высвобождение глутамата и субстанции Р из нервных окончаний. При этом активируются NMDA-рецепторы.
В результате блокады габапентином α2δ-субъединиц снижается вхождение ионов кальция в нервные окончания и высвобождение нейротрансмиттеров. Таким образом, ан-тигиперальгезивный эффект габапентина обусловлен снижением глутаминергической передачи на уровне спинного мозга. Другие клеточные механизмы, вероятно участвующие в реализации анальгетического эффекта габапентина, включают воздействие на NMDA-рецепторы, Na+-каналы, моноаминергиче-ские проводящие пути и опиоидную систему.
Помимо прямого анальгетического действия, габапентин обладает способностью предотвращать развитие острой толерантности к опиоидам, а также устранять уже развившуюся толерантность. В частности, в экспериментальных условиях было продемонстрировано свойство габапентина подавлять развитие антиноцицептивной толерантности к морфину [13].
Чаще всего препарат назначают однократно, за 1–2 часа до операции per os. Поскольку при увеличении дозы биодоступность препарата снижается, особенно актуальным является вопрос выбора оптимальной дозы. В исследовании, выполненном у пациентов, перенесших задний поясничный спондилодез [11], средняя эффективная анальгетическая доза габапентина (ED50) составила 21,7 мг/кг (от 19,9 до 23,5 мг/ кг). Согласно другим данным, превентивная доза габапентина 5 мг/кг у пациентов, перенесших дискэктомию на поясничном уровне, снижала послеоперационную потребность в фентаниле на 35% в течение 24 часов [23]. Различие в дозах, очевидно, объясняется различной травматичностью хирургических вмешательств. Еще в одном исследовании у пациентов, также оперированных на позвоночнике (преимущественно, дискэктомии), 38% снижение потребности в морфине было достигнуто однократным предоперационным введением габапентина в дозе 16 мг/кг [33].
Целесообразно ли назначение габапентина пациентам, послеоперационное обезболивание которых осуществляется с использованием тех или иных вариантов регионарной анальге- зии? Назначение 1200 мг габапентина до операции и в течение 2-х суток после нее достоверно снижало интенсивность послеоперационной боли у пациентов, оперированных на нижних конечностях, которым проводилась послеоперационная эпидуральная анальгезия (ЭА), в сравнении с теми, кто получал ЭА «в чистом виде» [34]. Потребность в ЭА была существенно ниже в группе габапентина спустя 24, 48 и 72 часа после операции. У пациентов группы габапентина отмечалась меньшая выраженность моторной блокады и большая удовлетворенность качеством послеоперационного обезболивания.
Сочетание габапентина с регионарной анестезией/аналь-гезией особенно показано в тех случаях, когда зона действия местного анестетика не перекрывает всей площади вероятного формирования гиперальгезии. В этой ситуации габапентин за счет центрального действия способен в определенной степени препятствовать формированию вторичной гиперальгезии и, следовательно, способствовать снижению интенсивности боли.
Имеется ряд клинических подтверждений эффективности габапентина в предупреждении формирования ХПБС. В частности, назначение 1200 мг препарата за 2 часа до резекции щитовидной железы в условиях общей анестезии с блокадой шейного сплетения позволило снизить частоту нейропатических болей жгучего характера через 6 месяцев после операции с 28% в группе сравнения до 4% [4].
Изучали возможность предупреждения развития как острого, так и хронического постмастэктомического болевого синдрома при помощи мультимодальной анальгезии, включающей 400 мг габапентина вечером накануне операции, а затем через каждые 6 часов до 8-х послеоперационных суток, а также нанесение крема EMLA вокруг операционной раны в течение 3-х суток и орошение элементов плечевого сплетения в подмышечной области 10 мл 0,75% ропивакаина во время операции [12]. В группе мультимодальной анальгезии требуемые дозы анальгетиков в послеоперационном периоде были приблизительно в 2 раза ниже, чем в группе сравнения. Хронические болевые ощущения через 3 месяца после операции отмечали в основной группе 46% пациенток после секторальной резекции железы и 44% после мастэктомии, в группе сравнения – 82% и 80% соответственно. Через 6 месяцев после операции средняя частота хронической боли в основной группе составляла 30%, в группе сравнения – 57%.
Однако есть и негативные данные. У пациенток, перенесших абдоминальную гистерэктомию, назначение 1200 мг габапентина за 1 час до операции, а затем в той же дозе однократно в течение 2-х суток не приводило к снижению частоты появления болевых ощущений (4–8%) в течение 3-х месяцев наблюдения, в сравнении с контрольной группой [34].
Актуальным является вопрос комбинированного назначения габапентина с анальгетиками, обладающими иными механизмами действия. С нашей точки зрения, перспективным (и практически неизученным) направлением представляется сочетанное применение габапентина и более мощных по анальгетическому эффекту неселективных НПВС.

Есть данные о том, что габапентин способствует сокращению сроков пареза кишечника после операций на органах нижнего этажа брюшной полости, в частности после абдоминальной гистерэктомии [34]. Механизмы данного эффекта не изучены.
Способность габапентина снижать частоту и выраженность тошноты впервые была выявлена у пациентов, которым проводилась химиотерапия. Предполагаемым механизмом является подавление активности нейротрансмиттера тахикинина. Определенную роль играет и опиоид-сберегающий эффект. Изучена способность габапентина снижать частоту послеоперационной тошноты и рвоты (ПОТР). У 250 пациентов, перенесших лапароскопическую холецистэктомию, назначение 600 мг габапентина за 2 часа до операции [23] достоверно снижало частоту ПОТР в сравнении с пациентами, которые получали плацебо (37,8% и 60% соответственно).
Данные доказательной медицины суммируют целесообразность использования габапентина для профилактики и лечения острого послеоперационного болевого синдрома.
Мета-анализ Tiippana E. с соавт. выявил целесообразность предоперационного однократного назначения габапентина для снижения интенсивности послеоперационной боли и потребности в анальгетиках [30]. В различных исследованиях было установлено, что назначение от 300 до 1200 мг габапентина снижает потребность в морфине от 20 до 60%.
Другой мета-анализ включил данные 18 исследований, представленных в 8 электронных базах данных (MEDLINE, EMBASE, PubMed и др.), за период с 1966 по март 2006 г. [24]. В 12 из них использовалась суточная доза 1200 мг. В 11 исследованиях габапентин назначался однократно за 1–2 часа до операции. В подавляющем большинстве исследований был подтвержден опиоид-сберегающий эффект габапентина в первые 24 часа после операции (в среднем – 35%), достоверное снижение интенсивности боли в покое на протяжении тех же 24 часов (от 7,2 до 14,3 мм по 100 мм ВАШ), а также при движении, от 8,2 до 10,2 мм (через 2,4 и 12 часов после операции).
Clivatti J. c соавт. осуществили анализ всех клинических рандомизированных исследований, выполненных в 2002– 2007 гг., и оценивавших влияние габапентина на формирование послеоперационного болевого синдрома [5]. Всего было включено 26 исследований (1020 пациентов). В 17 из них пациенты получали однократную дозу препарата (от 300 до 1200 мг) в интервале от 30 минут до 2-х часов перед операцией. В остальных исследованиях препарат назначали за 24 часа до операции и продолжали применять в течение 10 суток. Суточная доза составляла от 1200 до 1800 мг. Значимое снижение интенсив-ности боли было отмечено у 75% пациентов, получавших габапентин однократно и 55,6% получавших длительно. Потребление опиоидов было снижено у 82,4% пациентов при однократном приеме и 77,8% при длительном приеме препарата. Из побочных эффектов чаще всего отмечались головокружение и избыточная седация. Частота составила 5,9% и 5,9% при однократном приеме, 22,2% и 11,1% – при многократном соответственно.
Мы далеки от того, чтобы рассматривать габапентин в качестве идеального анальгетика для лечения послеоперационной боли. Более того, в инструкции к препарату купирование послеоперационной боли не значится среди показаний. Однако показанием является лечение нейропатической боли, а нейропатический компонент, как указывалось выше, зачастую представлен в структуре послеоперационной боли. В США габапентин является рекордсменом среди 160 наиболее часто назначаемых препаратов в отношении использования по показаниям, не указанным в инструкции.
Совокупность накопленных на сегодняшний день данных позволяет рекомендовать применение габапентина в дозе 900– 1200 мг за 1–2 часа до операции.
Сульфат магния
Среди препаратов, способных оказывать действие на NMDA-рецепторный комплекс, особый интерес вызывает сульфат магния, доступный любой российской клинике. Магний является четвертым по распространенности катионом человеческого организма. Он препятствует вхождению ионов Са2+ в клетку посредством неконкурентной блокады NMDA-рецепторов. Таким образом, магний выступает в роли физиологического антагониста кальция, оказывая влияние на различные потенциалзависимые кальциевые каналы, задействованные в механизмах ноцицепции [30]. Введение сульфата магния резко сокращает NMDA-опосредованные ионные потоки.
Первое клиническое исследование, показавшее, что перио-перационное внутривенное введение сульфата магния снижает потребность в анальгетиках в послеоперационном периоде, было выполнено M. Tramer c соавторами в 1996 г. [31]. При абдоминальной гистерэктомии пациентки в процессе индукции анестезии получали болюс 3 г с последующей инфузией со скоростью 2,5 мл/час в течение 20 часов. Это позволило снизить потребность в морфине на протяжении 2-х суток после операции с 91 мг в контрольной группе до 65 мг. Пациентки, получавшие магний, не имели нарушений сна в течение первых 2-х суток после операции.
В настоящее время установлено, что магния сульфат, как и другие антагонисты NMDA-рецепторов, потенцирует анальгетический эффект опиоидов, замедляет развитие острой толерантности к ним и снижает ее выраженность.
В ряде исследований было выявлено значительное снижение концентрации ионов магния в плазме пациентов как при обширных абдоминальных операциях, так и при менее травматичных вмешательствах, что, вероятно, и определяло их повышенную потребность в анальгетиках. Пациенты, перенесшие обширные операции, имеют более высокий риск развития гипомагниемии в первые 24 часа после операции [30]. Причинами считают повышенные потери магния с мочой, а также перемещение ионов Mg2+ между водными секторами. Снижение внеклеточной концентрации Mg2+ ниже физиологического уровня способствует значительному повышению реактивности NMDA-рецепторов. В условиях дефицита магния активируется процесс открытия NMDA-каналов под влиянием возбуждающих аминокислот глутамата и аспартата. Дополнительное назначение препаратов магния тормозит этот процесс. С учетом имеющихся данных об обратной зависимости между плазменной концентрацией Mg2+ и интенсивностью боли в родах, при инфаркте миокарда, панкреатите [36], профилактика периоперационной гипомагнезиемии представляется патогенетически обоснованной.
Большинство исследований посвящено изучению влияния магния на послеоперационную боль. Однако есть данные, что инфузия сульфата магния во время операции позволяет снизить требуемые дозы не только опиоидов, но и мышечных релаксантов, а также гипнотиков. Mg2+ и Ca2+ являются конкурентами за пресинаптические кальциевые каналы, что оказывает влияние на эффект различных препаратов для анестезии. Известно, что процесс высвобождения возбуждающих аминокислот, в котором задействованы пресинаптические кальциевые каналы, является одной из основных точек действия общих анестетиков. В эксперименте была продемонстрирована способность сульфата магния в дозозависимом режиме снижать МАК галотана. Кроме того, магний подавляет выброс ацетилхолина в моторных нервных окончаниях, потенцируя, таким образом, эффект миорелаксантов.
Очевидно, эффективность сульфата магния определяется рядом факторов: доза препарата и схема его назначения (болюс или длительная инфузия), тип операции (ее травматичность), исходная концентрация ионов магния в плазме. Предложены различные схемы назначения препарата: в/в болюсное однократное введение до начала хирургического вмешательства, болюс с последующей инфузией, инфузия без предварительного болюса.
Оптимальная доза магния для воздействия на NMDA-рецепторы пока не установлена. В редакционной статье, опубликованной в British Journal of Anaesthesia (2006), указано, что стандартная анальгетическая доза сульфата магния для пациента весом 70 кг составляет 2 г. [10]. В то же время в ряде исследований не было выявлено позитивного влияния на интенсивность боли и гораздо более высоких доз препарата.
Суммарное современное представление о роли сульфата магния в схемах мультимодальной анальгезии дает систематизированный обзор, в котором проанализированы результаты 14 рандомизированных контролируемых исследований (778 пациентов), посвященных изучению влияния магния на послеоперационную боль [32] .
В 9 исследованиях пациенты получали магния сульфат в виде пред- или интраоперационного болюса с последующей инфузией в течение 24 часов. Средняя суточная доза составляла 8,5 г (от 2,6 до 16,3 г). В 4 исследованиях (29%) было отмечено достоверное снижение интенсивности послеоперационной боли за период наблюдения от 7 до 24 часов. В 7 исследованиях (50%) не было выявлено влияния магния на послеоперационную боль в сравнении с плацебо. В 1 исследовании боль усиливалась на протяжении 3 часов наблюдения, еще в 2 работах интенсивность боли не оценивалась.
В 8 исследованиях (57%) было отмечено достоверное снижение потребности в анальгетиках на фоне назначения магния. В 4-х из них суммарное потребление морфина за период от 24 до 48 часов снижалось на 12–47% (в среднем – на 28%). Опиоид-сберегающий эффект соответствовал таковому при внутривенном введении кетамина или послеоперационном назначении НПВС.
Инфузия магния сопровождалась снижением частоты послеоперационной дрожи до 4,5%, в сравнении с 11,8% в контрольной группе. В 3-х исследованиях отметили улучшение качества сна пациентов в первую ночь после операции.
В 7 исследованиях оценивали плазменную концентрацию магния. В 5 из них у пациентов контрольной группы отметили достоверное снижение уровня магния в плазме на 9–27% от исходного (в среднем – на 11%). Следует отметить, что плазменная концентрация отражает лишь малую часть общего содержания магния в организме. Внутриклеточный уровень магния может быть низким, несмотря на нормальную концентрацию в плазме. Есть мнение, что способность магния снижать интенсивность боли и потребность в анальгетиках может быть обусловлена не прямым анальгетическим эффектом препарата, а профилактикой плазменной гипомагниемии, предупреждающей последующую активацию NMDA-рецепторов.
Известно, что инфузия сульфата магния может оказывать гипотензивный эффект за счет прямого вазодилятирующего действия, а также опосредованно, за счет симпатической блокады и снижения выброса катехоламинов. В то же время, ни в одном из вышеуказанных исследований не наблюдали нарушений гемодинамики, требующих медикаментозной коррекции.
Ограниченная информативность данного обзора обусловлена несколькими факторами. Прежде всего, большинство исследований включало малое число пациентов. В наиболее крупном исследовании (200 пациентов), не выявившем преимуществ назначения магния, вводился однократно болюс 4 г. [32]. В другом исследовании суточная доза превышала 16 г., при этом тоже не было установлено положительного влияния на интенсивность боли и потребность в анальгетиках [39]. С другой стороны, однократное введение болюса 3,6 г сопровождалось достоверным снижением интенсивности боли и потребности в анальгетиках у пациентов, перенесших ортопедические вмешательства. Единичные авторы анализировали эффективность использования наиболее перспективной, на наш взгляд, схемы назначения сульфата магния: нагрузочный болюс с последующей интраоперационной инфузией, пролонгированной на послеоперационный период.
Таким образом, в настоящее время нет однозначного мнения о целесообразности периоперационного применения магния с целью снижения интенсивности послеоперационной боли и снижения потребности в анальгетиках. Тем не менее, имеются предпосылки для дальнейшего изучения роли магния, поскольку есть серьезная биологическая основа рассматривать его в виде потенциального и многообещающего анти-ноцицептивного средства. Весьма вероятно, что он обладает синергизмом с другими антагонистами NMDA-рецепторов, в особенности с кетамином.

Заключение
В заключение хочется сказать, что перспективы использования и препаратов, воздействующих на NMDA-рецепторный комплекс, представляются нам весьма обнадеживающими. Залогом этого является их влияние на патофизиологические механизмы формирования острого и хронического послеоперационного болевого синдрома. Перспективны исследования, направленные на поиск рациональных сочетаний вышеуказанных препаратов и оптимальных схем их назначения в периоперационном периоде.
Список литературы Современные возможности безопиоидного послеоперационного обезболивания
- Apfelbaum J., Chen C., Mehta S. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged//Anesth. Analg. 2003. Vol. 97. P. 534-540.
- Argiriadou H., Himmelseher S., Papagiannopopulou P. Improvement of pain treatment after major abdominal surgery by intravenous S(+) ketamine//Anesth. Analg. 2004. Vol. 98. P. 14131418.
- Benhamou D., Berti M., Brodner G. Postoperative analgesic therapy observational survey (PATHOS): a practice pattern study in 7 central/southern European countries//Pain. 2008. Vol. 136. P. 134-141.
- Brogly N., Wattier J-M., Andrieu G. Gabapentin attenuates late but not early postoperative pain after thyroidectomy with superficial cervical plexus block//Anesth. Analg. 2008. Vol. 107. P. 1720-1725.
- Clivatti J., Sakata R., Issy A. Review of the use of gabapentin in the control of postoperative pain. Rev.Bras.Anesthesiol. 2009;.59:.87-98.
- Dahl J., Mathiesen O., Moniche S. Protective premedication: an option with gabapentin and related drugs. A review of gabapentin and pregabalin in the treatment of postoperative pain//Acta Anaesth. Scand. 2004. Vol. 48. P. 1130-1136.
- De Kock M., Lavand'homme P., Waterloos H. Balanced analgesia in the perioperative period: is there place for ketamine?//Pain. 2001. Vol. 92. P. 373-380.
- Dirks J., Fredensborg B., Christensen D. A randomizes study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy//Anesthesiology. 2002. Vol. 97. P. 560-564.
- Dolin S., Cashman J., Bland J. Effectiveness of acute postoperative management. I. Evidence from published data//Br. J. Anaesth. 2002. P. 409-423.
- Durieux M. Peripheral analgesic receptor system//Br. J. Anaesth. 2006. Vol. 97. P. 273-274.
- Elstraete A., Tirault M., Lebrun T. The median effective dose of preemptive gabapentin on postoperative morphine consumption after posterior lumbar spinal fusion//Anesth. Analg. 2008. Vol. 106. P. 305-308.
- Fassoulaki А., Patris K., Sarantopoulos C. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer//Anesth. Analg. 2002. Vol. 95. P. 985-991.
- Gilron I., Biederman J., Jhamandas K. Gabapentin blocks and reverses antinociceptive mor-phine tolerance in the rat paw-pressure and tail-flick tests//Anesthesiology. 2003. Vol. 98. P. 1288-1292.
- Hahm T., Ahn H., Bae C-D. Protective effects of gabapentin on allodynia and a261-subunit of voltage-dependent calcium channel in spinal nerve-ligated rats//J. Korean Med. Sci. 2009. Vol. 24. P. 146-151.
- Kehlet H., Rung G., Callesen T. Postoperative opioid analgesia: time for a reconsideration?//J. Clin. Anaesth. 1996. Vol. 8. P. 441-445.
- Kehlet H., Holte K. Effect of postoperative analgesia on surgical outcome//Br. J. Anaesth. 2006. Vol. 87. P. 62-72.
- Lavand'homme P., De Kock M., Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery//Anesthesiology. 2005. Vol. 103. P. 813-820.
- Laulin J.-P., Maurette P., Rivat C. The role of ketamine in preventing fentanyl-unduced hy-peralgesia and subsequent acute morphine tolerance//Anesth. Analg. 2002. Vol. 94. P. 1263-1269.
- Maier C., Nestler N., Richter H. The quality of postoperative pain management in German hospitals//Dtsch. Arstebl. Int. 2010. Vol. 107. P. 607-614.
- Mao J., Price D., Mayer D. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible mechanisms//Pain. 1995. Vol. 62. P. 259-274.
- McCartney C., Sinha A., Katz J. A qualitative systematic review of the role of N-methyl-D-aspartate receptor antagonists in preventive analgesia//Anesth. Analg. 2004. Vol. 98. P. 1385-1400.
- Oderda G., Evans S., Lloyd J. Cost of opioid-related adverse drug events in surgical pa-tients//J. Pain Symptom Manage. 2003. Vol. 25. P. 276-283.
- Pandey C., Navkar D., Giri P. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy: a randomized double-blind placebo-controlled study//J. Neurosurg. Anesthesiol. 2005. Vol. 17. P. 65-68.
- Peng P., Wijeysundera D., Li C. Use of gabapentin for perioperative pain control -a meta-analysis//Pain Res. Manage. 2007. Vol. 12. P. 85-92.
- Polomano R., Rathmell J., Krenzischer D. Emerging trends and new approaches to acute pain management//Pain Management Nursing. 2008. Vol. 9. P. 33-41.
- Rowbotham W. Gabapentin: a new drug for postoperative pain?//Br. J. Anaesth. 2006. Vol. 96. P. 152-155.
- Stubhaug A., Breivik H., Eide P. Mapping of punctuate hyperalgesia around a surgical inci-sion demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery//Acta Anaesth. Scand. 1997. Vol. 41. P. 1124-1132.
- Subramaniam K., Subramaniam B., Steinbrook R. Ketamine as adjuvant analgesic to opioids: a quantitative an qualitative systematic review//Anesth. Analg. 2004. Vol. 99. P. 482-495.
- Suzuki M., Haraguti S., Sugimoto K. Low-dose intravenous ketamine potentiates epidural analgesia after thoracotomy//Anesthesiology. 2006. Vol. 105. P. 111-119.
- Tiippana E., Hamunen K., Kontinen V. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety//Anesth. Analg. 2007. Vol. 104. P. 1545-1556.
- Tramer M, Schneider J., Marti R., Rifat K. Role of magnesium sulfate in postoperative analgesia//Anesthesiology. 1996. Vol. 84. P. 340-347.
- Tramer M., Glynn C. An evaluation of a single dose of magnesium to supplement analgesia following ambulatory surgery: randomized controlled trial//Anesth. Analg. 2007. Vol. 104. P. 1374-1379.
- Turan A., Karamanlioglu B., Memeis D. Analgesic effect of gabapentin after spinal surgery//Anesthesiology. 2004. Vol. 100. P. 935-938.
- Turan A., Kaya G., Karamanlioglu B. Effect of oral gabapentin on postoperative epidural analgesia//Br. J. Anaesth. 2006. Vol. 96. P. 242-246.
- Urban M., Ya Deau J., Wukovits B. Ketamine as an adjunct to postoperative pain man-agement in opioid tolerant patients after spinal fusions: a prospective randomized trial//HSS J. 2008. Vol. 4. P. 62-65.
- Weissberg N., Schwartz G., Shemesh O. Serum and intracellular electrolytes in patients with and without pain//Magnes. Res. 1991. Vol. 4. P. 49-50.
- Weinbroum A. A single small dose of postoperative ketamine provides rapid and sustained improvement in morphine analgesia in the presence of morphine-resistant pain//Anesth. Analg. 2003. Vol. 96. P. 789-795.
- Wheeler M., oderda G., Ashburn M. Adverse events associated with postoperative opiod analgesia: a systemic review//J. Pain. 2002. Vol. 3. P. 159-180.
- Zarauza R., Saez-Fernandez A., Iribarren M. A comparative study with oral nifedipine, in-travenous nimodipine and magnesium sulfate in postoperative analgesia//Anesth. Analg. 2000. Vol. 91. P. 938-943.


