Старческая астения и старческая апатия в повседневной клинической практике в условиях пандемии новой коронавирусной инфекции COVID-19
Автор: Веселова Дарья Константиновна, Белопасов Владимир Викторович
Журнал: Клиническая практика @clinpractice
Рубрика: Обзоры
Статья в выпуске: 1 т.13, 2022 года.
Бесплатный доступ
В обзорной статье освещены патогенез, клинические проявления, диагностические критерии старческой астении и старческой апатии у пожилых людей. Особое внимание уделено феномену саркопении: рассматриваются фенотипическая классификация и современные подходы к лечению. Знание и понимание основных патогенетических звеньев старческой астении и старческой апатии, а также разработка единого алгоритма лечения данных патологических состояний могут оказать значительное влияние на продолжительность и качество жизни пожилых людей.
Старческая астения, старческая апатия, саркопения, новая коронавирусная инфекция, иммуносенесценция, covid-19
Короткий адрес: https://sciup.org/143178545
IDR: 143178545
Текст обзорной статьи Старческая астения и старческая апатия в повседневной клинической практике в условиях пандемии новой коронавирусной инфекции COVID-19
Проблемы старения волновали людей во все времена. Новая эпоха диктует новые правила: в наше время слово «долголетие» — отнюдь не пустой звук, здоровое старение является важным приори- тетом общественного здравоохранения. Очевидно, что снижение физиологического резерва органов и систем, ведущее к повышению уязвимости организма пожилого человека к воздействию триггерных факторов, сопряжено с высоким риском
Лицензия CC BY-NC-ND 4 / The article can be used under the CC BY-NC-ND 4 license
развития неблагоприятных исходов для здоровья, в частности потери автономности, инвалидизации, преждевременной когнитивной дисфункции. Таким образом, своевременное предотвращение истощения внутренних резервов организма имеет решающее значение для достижения фундаментальных целей здорового старения, а именно для развития и поддержания функциональных возможностей, обеспечивающих благополучие людей пожилого и старческого возраста.
Систематизация данных многочисленных публикаций, посвященных старению, позволила выделить ряд проблем, стоящих на повестке дня и требующих пристального внимания. К ним можно отнести старческую астению, история изучения которой уходит корнями всего лишь в 1943 год, когда в Британском медицинском журнале была впервые опубликована статья Марджори Уоррен (Marjory Warren) [1], стоявшей у истоков гериатрической медицины. Автор предложила ввести в клиническую практику персонализированный подход к пожилым людям, исходя из их специфических потребностей, значительно отличающихся от таковых в более молодом возрасте. Именно новая система классификации пациентов пожилого и старческого возраста позволила внедрить полезные виды вмешательств для различных групп больных, а также значительно преобразить качество оказываемой им врачебной помощи и сестринского ухода.
Термин «старческая астения» (англ. frailty — хрупкость), характеризующий угасание физиологических функций, развитие когнитивного дефицита, гиподинамии у пожилых людей, впервые стал использоваться в научных статьях с 1974 г., когда Чарльз Ф. Фэйхи (Charles F. Fahey), выступая на Федеральном совете по проблемам старения (Federal Council on Aging) Соединенных Штатов Америки, ввел его для оценки пожилых людей со «значительными физическими, когнитивными и эмоциональными нарушениями, нуждающихся в дополнительном внимании» [2, 3]. В 1978 г. он включил дополнение, что это «лица старше, но не всегда, 75 лет, которые в связи с наличием у них различных хронических заболеваний часто требуют одной или нескольких вспомогательных услуг для того, чтобы справиться с повседневной жизнью» [4]. Почти аналогичный взгляд высказал в своем научном обзоре под названием «Люди со старческой астенией — кто они?» (Who are the frail elderly?) K.W. Woodhouse [5]. По его мнению, «это люди в возрасте старше 65 лет, нуждающиеся в посторонней помощи для выпол- нения повседневных задач, зачастую находящиеся на попечении в домах престарелых, не страдающие явными заболеваниями внутренних органов».
Особенно значительный рост публикаций по данной проблеме наблюдался в 90-х годах прошлого века. В ходе дискуссий термин «старческая астения» неоднократно пересматривался. D. Buchner и E. Wagner [6] высказана альтернативная точка зрения: это «обратимое состояние, характеризующееся снижением общего физиологического резерва с сохранением способности выполнять сложные функциональные задачи».
В клинических рекомендациях Российской ассоциации геронтологов и гериатров предлагается следующее определение [7]: «Старческая астения — ключевой гериатрический синдром, характеризующийся возрастассоциированным снижением физиологического резерва и функций многих систем организма, приводящий к повышенной уязвимости организма пожилого человека к воздействию эндо-и экзогенных факторов и высокому риску развития неблагоприятных исходов для здоровья, потери автономности и смерти», он чаще прогрессирует, чем регрессирует. Причиной является наличие или развитие мультикоморбидных форм патологии.
Социальная и медицинская значимость своевременных диагностики и лечения возникающих и прогрессирующих с годами у пожилых людей заболеваний очевидна. По демографическим оценкам Организации Объединенных Наций и Вашингтонского Университета, с каждым годом отмечается прирост населения пожилого и старческого возраста, и, согласно прогнозам, в ближайшее время старение населения мира ускорится [8]: к 2050 г. 22% населения Земли будут составлять люди пенсионного возраста, а в странах мира на каждого работающего гражданина будет приходиться по пенсионеру. В связи с этим прогнозируется и рост числа людей с синдромом старческой астении. На данный момент распространенность данной формы патологии по всему миру среди населения в возраcте старше 60 лет оценивается примерно в 11–16%, значительно выше показатели у лиц в возрасте старше 85 лет. Во многих публикациях подчеркивается, что данный синдром чаще встречается у женщин [9–11].
СОВРЕМЕННЫЙ ВЗГЛЯД НА ПРОБЛЕМУ СТАРЧЕСКОЙ «ХРУПКОСТИ»
Предлагаются к рассмотрению две модели развития старческой астении. Фенотипическая модель, предложенная L. Fried и соавт. [12], рассматривает
КТ ГА
старческую астению в качестве синдрома регресса физиологического резерва, диагностическими критериями которого являются быстрая утомляемость, спонтанное нерезкое снижение веса в течение 12 мес, снижение мышечной силы, ограничение физической активности, замедление скорости ходьбы. Выявление трех и более симптомов, согласно фенотипической модели проекта SHARE (Survey of Health, Ageing and Retirement in Europe; Обзор состояния здоровья, старения и выхода на пенсию в Европе), подразумевает наличие у пациента старческой астении, двух и менее — старческой преастении. Но такой подход к оценке клинических проявлений, по нашему мнению, является упрощенным, так как остаются без внимания значимые маркеры старческой астении — нарушения эмоционального состояния, когнитивная дисфункция и другие проявления дисрегуляции ментальной сферы [13].
В последние годы большие усилия прилагаются к выявлению внутриклеточных механизмов, лежащих в основе старческой астении. Ряд зарубежных авторов отводит важную роль в инициации клинических симптомов «поломке» микроРНК (miRNA, microRNA) [14]. МикроРНК, являясь одним из двух основных классов низкомолекулярных РНК, участвуют в регуляции экспрессии генов с помощью механизма РНК-интерференции и являются ключевыми трансрегуляторами большинства физиологических процессов: вот почему нарушения регуляции микроРНК констатируют при множестве заболеваний, в том числе при старческой астении, когнитивном дефиците различной степени выраженности [15]. Более того, некоторые микроРНК, по-видимому, играют важную роль в ингибировании апоптоза нервных клеток, пролиферации, дифференцировке и выживании нейронов [16].
Анализ зарубежных публикаций [4, 17] дает понимание того факта, что микроРНК в перспективе могут стать не только диагностическими, но и прогностическими биомаркерами старческой астении, могут быть использованы как для оценки скрытых механизмов развития и течения данного синдрома, так и в качестве потенциальных терапевтических агентов.
Модель накопления дефицитов была впервые представлена в статье A.В. Mitnitski и соавт. [18]: авторы рассматривают старческую астению как дефицитарное состояние, форму отклонения от возрастной нормы, которое проявляется не только клинически, но и в результатах лабораторных и/или функциональных тестов. Каждый признак получает цифровое выражение, при математической обработке полученных данных вычисляется индекс (Frailty Index, FI), величина которого позволяет объективно определить степень выраженности дефицита.
Мы — сторонники недавно предложенной концепции A.L. Cardoso и соавт. [4], которые считают, что при диагностике возникающих отклонений в соматической и психической сфере должны учитываться не только результаты гериатрической оценки (comprehensive geriatric assessment, CGA), немаловажное значение в их генезе могут играть присущие каждому индивидууму, но недооцененные им и врачом генетические, биологические, медицинские, социально-средовые неблагоприятные воздействия (табл. 1).
Предложено большое количество разнообразных шкал, опросников и тестов, определяющих наличие и степень выраженности синдрома старческой астении: Fried Frailty, FRAIL Scale, SHARE Frailty Instrument 75+, Frailty Phenotype, Continuous Frailty Scale (CFS), Frail Non-Disabled Scale, SOF Frailty, Frailty Trait Scale (FTS), SARC-F, SARC-CalF, SHARE Frailty, UEF Frailty, INTER-FRAIL, PRISMA-7, IMSIFI, LUCAS, Modelo Fried adaptado, Groningen Frailty Indicator, Tilburg Frailty Indicator, Gerontopole. Несмотря на то, что тесты не валидизированы в нашей стране, некоторые из них хорошо зарекомендовали себя в гериатрической практике. Помимо отечественного опросника «Возраст не помеха», предпочтение отдается более простым, повсеместно доступным методам, таким как тест 6-минутной ходьбы, фиксирование времени прохождения 4 м, вставания со стула 5 раз без помощи рук или подсчет количества подъемов в течение 30 сек. Каждый из них достаточно надежен, особенно, если в показатели измерения внесены поправки на рост и массу тела больного [19]. Для скрининговой оценки востребованы тест «Встань и иди» (Timed Up and Gotest, TUG), опросники PRISMA-7, SARC-F, в которых пациенту предлагается оценить степень снижения мышечной силы, скорости ходьбы, вставания со стула, подъема по лестнице, эпизоды падений. Диагностически значимым считается результат ≥4 баллов [19]. При использовании теста на устойчивость (стопы вместе, полутандемное, тандемное положение ног в течение 10 сек), шкал баланса Берга (BBS), ходьбы и равновесия Тинетти (Functional Mobility Assessment in Eldery Patients), падений Морзе (Morse Fall Scale), краткой батареи тестов физической активности (The Short Physical Performance Battery, SPPB) оценивается способ-
Таблица 1 / Table 1
Признаки, указывающие на вероятное наличие синдрома старческой астении [19] / Signs indicating the probable presence of senile asthenia syndrome [19]
|
Медицинские |
|
|
Когнитивноповеденческие |
|
|
Функциональные |
|
|
Медикаментозные |
• факторы, предрасполагающие к развитию нежелательных реакций на лекарственные средства (наличие ≥4 хронических заболеваний, хроническая сердечная недостаточность, заболевания печени, полипрагмазия, анамнез нежелательных побочных реакций) |
|
Социально-средовые |
|
Примечание. * Признаки, свидетельствующие о более высокой вероятности наличия у пациента синдрома старческой астении.
Note: * Signs indicating a higher probability of the presence of senile asthenia syndrome.
ность удерживать равновесие в течение 10 сек, скорость ходьбы на расстояние 4 м и время (в сек), сохранение баланса при пятитикратном подъеме со стула без помощи рук (возрастной показатель нормы — 10–12 баллов, 8–9 баллов — преастения, ниже — старческая астения).
По мнению экспертов EWGSOP2 [1], можно ограничиться оценкой одного параметра, который при диагностике саркопении является основным, — силы сжатия кисти с помощью кистевого динамометра Джамара. Стандартный вариант: тестирование правой и левой руки проводится трехкратно. Подтверждением саркопении служит снижение показателей мышечной силы менее 20 кг для женщин и менее 30 кг для мужчин [20, 21]. Однако при ряде заболеваний из-за нарушения подвижности в суставах кисти динамометрия неприемлема.
Изменение физического статуса больного констатируется при клиническом, инструментальном, лабораторном обследовании. Не менее ценная информация может быть получена при анализе шкал общего клинического впечатления (Clinical Global Impression Scale, CGI), FRAIL (Fatigue, Resistance, Ambulation, Illnesses, Loss of Weight), оценки здоровья (Patient Health Questionnaire, PHQ-9), статуса (шкала SPICES) и питания пациента (Mini Nutritional assessment, MNA). Базовую функциональную активность определяют по индексу Бартела (Barthel Activities of daily living Index), инструментальную — по шкале Лоутона (Lawton Instrumental activities of daily living Scale), степень выраженности немощности — по шкале оценки потребности и объема социально-бытовой помощи и ухода, когнитивного дефицита — по краткой шкале оценки психического статуса (Mini Mental State Examination, MMSE), Монреальской шкале оценки когнитивных функций (Montreal Cognitive Assessment, MoCA) [22, 23].
САРКОПЕНИЯ
Cаркопения (от греч. sarx — плоть, тело , penia — потеря ), ассоциированное с возрастом и/или наличием органной или системной патологии
КТ ГА
прогрессирующее заболевание мышечной ткани, имеющее в МКБ (ICD)-10 код М62.8, было выделено в качестве отдельной нозологии в 1989 г. I. Rosenberg [24], в 2019 г. достигнут консенсус по использованию критериев и методов его диагностики [1]. Основные признаки — снижение мышечной силы, мобильности; нарушение автоматизма, скорости исполнительных функций при смене положения и форм движения; латентно нарастающая диффузная гипотрофия/ атрофия мышц; потеря веса и мышечной массы; немощность; неустойчивость; потеря равновесия, особенно при ходьбе; высокий риск травматизма, переломов и других осложнений. При динапе-нии (пресаркопении) больной субъективно отмечает снижение мышечной силы в тесте динамометрии, независимо от гендерной принадлежности. Другие признаки поражения, функционирования мышц отсутствуют, общая мышечная масса не претерпевает изменений. Развитие данного осложнения связано с возрастзависимым гормональным дисбалансом — уменьшением уровня общего тестостерона, соматотропного гормона, инсулиноподобного фактора роста, повышением паратиреоидного гормона [17].
В патоморфологическом исследовании мышечных биоптатов при естественном старении [25] установлено значительное уменьшение поперечного сечения быстрых мышечных волокон, при этом размер медленных остается неизменным. Возраст- ная редукция мышечных волокон сопровождается их денервацией, уменьшением числа митохондрий в мышечных и нервных клетках, компенсаторным перераспределением нагрузки на действующие миоциты, изменениями в нейромышечных синапсах в виде транссинаптической дегенерации [26, 27].
Результаты ЭМГ-исследования у пожилых свидетельствуют об уменьшении числа функционирующих моторных единиц, особенно страдают самые крупные и «быстрые» мышечные волокна [27].
У больных саркопенией выраженность морфологических и нейрофизиологических изменений неуклонно возрастает, особенно при нейродеге-неративных заболеваниях (болезнь Паркинсона, болезнь Альцгеймера, боковой амиотрофический склероз) [28–31]. Их наличие и тем более прогрессирование негативно отражаются на трудоспособности, качестве жизни, становятся причиной развития инвалидности и преждевременной смертности [32, 33]. Согласно статистическим данным, число больных саркопенией неуклонно увеличивается.
В зависимости от причины выделяют первичную (возрастассоциированную — у 10–29% старше
60 лет) и вторичную (обусловленную гиподинамией, особенностями питания, образа жизни, приемом некоторых лекарственных препаратов, перенесенными и сопутствующими формами патологии — СПИД, заболевания/опухоли эндокринных желез, легких, сердца, почек, соединительной и мышечной ткани, ЦНС) саркопению [30, 31, 34–37]. В последние годы получены доказательства связи утраты мышечной массы с генами, принимающими участие в инициировании и развитии патологического процесса ( ACE, ACTN3, MSTN, CNTF, IGF1, VDR ), генами-кандидатами ( GREM1, TRHR, ACVR1B ) [38].
Патогенетические механизмы развития саркопе-нии активно изучаются, большое значение придается характерному для старения возрастзависимому снижению уровня тестостерона, эстрогена, соматотропного гормона, особо подчеркивается роль витамина D, инсулиноподобного фактора роста-1, инсулина, лептина [39].
В последние годы доминирует представление, что хроническое персистирующее воспаление, окси-дативный стресс [40] и нейромышечная дегенерация [41] являются основными причинами поражения скелетной мускулатуры, обусловливающими снижение мышечной массы и силы. Оба признака — главные атрибуты старческой астении. Возникшие у больного нарушения в системе гомеостаза могут обусловить развитие миопении [32], миостеастоза [42], саркопе- нического, остеосаркопенического ожирения, остео-саркопении (снижение мышечной и костной массы) [43–46]. Каждому из указанных фенотипов присущи свойственные только ему клинические и морфологические проявления. Патогномонично усиление выработки провоспалительных цитокинов, снижение чувствительности мышц к инсулину [47, 48].
Подтверждением диагноза считается потеря мышечной массы, снижение мышечной силы в сочетании с одним из двух признаков (уменьшение объема мышечной массы, нарушение функции скелетной мускулатуры) [1]. Обнаружение отклонений по всем трем параметрам — повод, чтобы заподозрить вероятность саркопении [49]. Для скрининга используют тесты «Ishii’s formula» (оценка возраста, силы сжатия кисти, окружности голени), Chair Stand Test [11], опросники SARC-F, SARC-CalF, MSRA-5, MS-RA-7 (Mini Sarcopenia Risk Assessment) и тест Short Physical Performance Battery (SPPB). Определение мышечной силы скелетной мускулатуры чаще всего проводят с помощью ручного динамометра. У пациентов с выраженной атаксией, страдающих деменцией, имеющих тяжелую сердечно-сосуди- стую патологию [1], можно ограничиться измерением скорости ходьбы, где снижение показателей <0,8 м/с служит критерием наличия у больного тяжелой саркопении [50].
Инструментальное измерение силы мышц нижних конечностей и мышц, выпрямляющих позвоночник, компьютерная томография (КТ) с определением скелетно-мышечного индекса, рентгеновская абсорбциометрия, изокинетическая динамометрия, биоимпедансометрия используются редко, преимущественно при проведении клинических исследований [33, 51]. Ценная информация для объективизации полученных данных может быть получена при использовании магнитно-резонансной томографии и ультразвукового исследования [52–54]. Внесение в протокол ультразвукового обследования изучение мышечной ткани — хорошее подспорье при обследовании маломобильных пациентов.
СТАРЧЕСКАЯ АПАТИЯ
Термин «апатия» (от греч. а — отсутствие , pathos — страсть, волнение ) обозначает симпто-мокомплекс, проявляющийся в безразличии, безучастности, отрешенности к происходящему, отсутствии эмоционального реагирования и стремления к какой-либо деятельности. Главной особенностью апатии является дефицит мотивации, а в отличие от депрессии ей характерна эмоциональная нейтральность, но не гипотимия. Чаще всего апатия является спутником развития тяжелых, нередко малокура-бельных заболевний, в том числе неврологических, таких как болезнь Паркинсона, рассеянный склероз, боковой амиотрофический склероз, все виды деменции [55, 56]. При заболеваниях с хорошим прогнозом наличие апатии нельзя связать с нозологией, объяснить изменением уровня сознания или когнитивными нарушениями [57]. Обращают внимание на отсутствие у этих больных самоинициированных реакций [58], но эмоциональная нейтральность не означает, что больной не в состоянии эмоционально реагировать на внешние воздействия [59].
R. Levy и B. Dubois [60], изучая механизмы возникновения апатии, выделили три патогенетических процесса, определяющих ее развитие:
-
1) эмоционально-аффективный (при нарушении взаимосвязи между эмоционально-аффективными сигналами при предстоящем или текущем патологическом процессе с повреждением наиболее важных структур — префронтальной коры и/или базальных ганглиев, определяющих инициацию и протекание поведенческих реакций);
-
2) когнитивный (в связи с возникшими проблемами в разработке плана текущих или предстоящих действий);
-
3) снижение самоактивации (из-за невозможности активировать мысли или действия при относительно сохранной способности реагировать на побуждающие стимулы извне). Этот вариант авторы назвали «психической акинезией».
В 2009 г. группой французских ученых были разработаны и предложены к использованию в клинической практике диагностические критерии апатии:
-
1) характеризующий ее главный признак — отсутствие мотиваций — должен присутствовать не менее 4 недель;
-
2) обязательное наличие двух из трех других симптомов — дефицит целенаправленной когнитивной активности, ограничение форм целенаправленного поведения, снижение уровня эмоционального реагирования;
-
3) отстранение от бытового и социального функционирования [61].
Выявление диагностических критериев апатии облегчается с использованием шкалы Apathy Evaluation Scale (AES) [62].
В 2018 г. были определены новые диагностические стандарты. Согласно обновленным критериям, наличие апатии у пациента достоверно подтверждается [63, 64]:
-
1) количественным снижением целенаправленной активности во всех сферах жизнедеятельности (эмоциональной, поведенческой, когнитивной, социальной) по сравнению с предыдущим уровнем функционирования. О возникших проблемах может сообщить сам пациент или наблюдающие за ним лица;
-
2) выявлением по крайней мере двух симптомов из трех доменов, характеризующих функционирование, при условии, что они присутствуют у пациента большую часть времени на протяжении как минимум последних 4 недель:
-
• домен I (поведение, память)1: привлекающие внимание окружающих изменения в целенаправленной деятельности и познавательной активности, о чем свидетельствует один и более из следующих симптомов: — общий уровень активности (пациент недостаточно активен в повседневной дея-


тельности, не прилагает должных усилий при выполнении необходимых задач или нуждается в побуждении);
-
— настойчивость (отсутствуют посылы к общению, выполнению рутинной физической и умственной работы, решению возникших проблем, поиску способов, как справиться с ними);
-
— реализация выбора (большую часть времени пациента занимает выбор между вопросами делать или не делать);
-
— интерес к посторонним проблемам (утрачивается или значительно снижается интерес к происходящим событиям);
-
— интерес к собственной личности (утрата или значительное снижение интереса к собственным здоровью, внешнему виду, нуждам и потребностям);
-
• домен II (эмоциональная сфера)1: отсутствие или ослабление эмоциональной реактивности выражается в наличии по крайней мере одного
из следующих симптомов:
-
— эмоциональные реакции на позитивные и негативные события не столь выражены, как это было до заболевания;
-
— пациент реже проявляет самогенерируе-мые эмоции, не заинтересован в общении; события, которые должны быть для него значимы, остаются без должного внимания;
-
— утрачивается сопереживание: пациент не беспокоится о том, как его слова и поступки влияют на окружающих; снижается заинтересованность в идентификации себя, общении другими людьми, в том числе значимыми;
-
— угасают вербальные или физические реакции, отражающие эмоциональное состояние пациента;
-
• домен III (социальная сфера): отсутствие или ослабление вовлеченности в социальное взаимодействие, на что указывает по крайней мере один из следующих симптомов:
-
— пациент не проявляет инициативы в реализации планов на социальные или досуговые мероприятия;
-
— он неохотно участвует либо полностью равнодушен к социальным и досуговым мероприятиям, предлагаемым ему окружающими людьми;
-
— меньше интересуется членами своей семьи, работой, друзьями;
-
— не склонен начинать сам разговор или старается как можно быстрее отстраниться от общения;
-
— самоограничивается от социальных контактов: предпочитает оставаться дома чаще или дольше обычного; не проявляет интереса к знакомству с другими людьми. Совокупная оценка выделенных доменов с использованием мини-теста психического состояния, Монреальского когнитивного теста (MoCA), батареи оценки функций лобных долей (FAB), шкалы депрессии Гамильтона, обновленных версий клинической диагностики апатии (AES-C, AES-S) позволяет охарактеризовать данный признак как гетерогенный синдром потери мотивации, изменения форм аффекта, поведения, исполнительных функций и уровня познания, связанный с возможным повреждением головного мозга [59, 62].
При проведении позитронно-эмиссионной томографии головного мозга установлено, что у пациентов с ранним началом болезни Альцгеймера появление апатии ассоциировано со значительным снижением метаболизма глюкозы в левых лобноорбитальных областях [65]. Предполагается, что ее возникновение является следствием патологических изменений в орбитофронтальной коре, передней поясной извилине и миндалевидном теле височной доли, у больных с когнитивной апатией — преимущественно в латеральной задней лобной коре, с двигательной апатией — в полосатом теле, педункулопонтийном ядре (PPN) [66, 67].
B.V. Chilovi и соавт. [68], обследуя больных с умеренными когнитивными расстройствами, апатией, депрессией и их сочетанием, обнаружили самый высокий процент перехода в деменцию при наличии апатии (60%), меньший (19%) — если имелась депрессия и апатия. К аналогичному выводу при оценке 1713 пациентов пришел М. Ruthirakuhan [69]: пациенты с апатией имеют больший риск развития деменции, чем с депрессией. Эти результаты нашли подтверждение и в аналитическом обзоре [70], и публикации N. Roberto и соавт. [71]. При исследовании 2137 пациентов было установлено, что из 4 форм эмоционального реагирования (раздражительность, апатия, тревога и депрессия) только аффективная неустойчивость и апатия являются предикторами деменции; тревога и депрессия не относятся к факторам риска. Характерный для апатии симптомокомплекс может быть идентифицирован даже на стадии развития легких когнитивных нарушений [59, 62].
Ранняя оценка клинических проявлений и выявление апатии практически значимы для прогнозирования возможной эволюции от умеренного когнитивного расстройства к деменции не только при заболеваниях головного мозга, но и при наличии у больных старческой астении.
СИНДРОМ СТАРЧЕСКОЙ АСТЕНИИИ COVID-19
С начала пандемии люди пожилого и старческого возраста составили наибольшую долю населения по результатам госпитализации и смертности из-за COVID-19 не только в нашей стране, но и в Европе, Великобритании, Соединенных Штатах Америки (США) и Канаде [9, 10]. По сравнению с молодыми, они более подвержены неблагоприятным исходам не только при вирусных инфекциях, но и многих сопутствующих возрасту заболеваниях [25]. Установлено, что с годами у пожилых людей происходит постепенное подавление иммунной реактивности из-за снижения количества нативных CD4+CD45RA+Т-лимфоцитов, накопления Т-клеток памяти и изменения функции В-клеток, продуцирующих антитела [72, 73]. Такая трансформация, являющаяся ключевым звеном инфламэйджинга (от англ. inflammageing), получила название «им-муносенесценция» (от англ. immunosenescence). Теория инфламэйджинга рассматривает старение организма и развитие возрастассоциированных заболеваний как следствие хронического персистирующего воспалительного процесса, который развивается на протяжении всей жизни под негативным воздействием факторов инфекционной и неинфекционной природы. Инфламэйджинг обладает рядом свойств, отличающих его от острого воспаления, а именно: хроническое течение, слабая степень вы- раженности воспалительного процесса, стертость клинических проявлений [7]. Примечательно, что инфламэйджинг ассоциируется с прогрессирующим увеличением провоспалительных цитокинов, включая интерлейкины IL-1b, IL-6, фактор некроза опухоли альфа (TNF-α) [74], что существенно влияет на исход заболевания при заражении COVID-19 и выживаемость пациентов с синдромом старческой астении при тяжелом течении инфекции [74].
Обобщенные данные по динамике развития патологического процесса при инфицировании COVID-19 у пожилых с отсутствием и наличием старческой астении представлены на рис. 1.
Получены также убедительные доказательства [75], что снижение разнообразия кишечной микробиоты у пациентов пожилого и старческого возраста также является одним из патологических звеньев развития тяжелой формы COVID-19.
Хотя наличие синдрома старческой астении не определяет повышенный риск заражения новым коронавирусом тяжелого острого респираторного синдрома 2 (SARS-CoV-2) [76], тем не менее у пожилых людей, имеющих данный симптомокомплекс, вероятность тяжелого течения COVID-19, включая развитие дыхательной недостаточности и тяжелой гипоксемии, с необходимостью применения искусственной вентиляции легких выше, чем у пациентов с преастенией и отсутствием астении [77, 78].
Метаболический стресс, инициированный «цито-киновым штормом», недостаточность потребления белка, длительная иммобилизация, наличие ассоциированной с возрастом и инфицированием поли-органной патологии, гипо- и адинамия, нарушение энергообеспечения мышечной ткани являются значимыми факторами развития и прогрессирования миостеатоза и саркопении не только у больных стар-
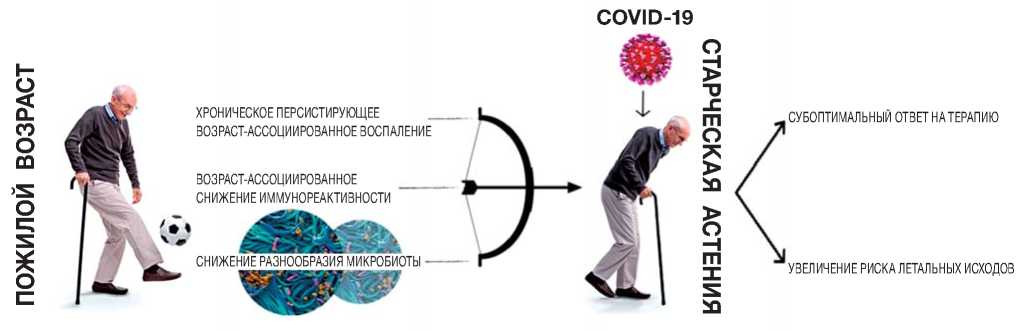
Рис. 1. Ключевые патологические звенья, определяющие неблагоприятный исход COVID-19 у пациентов с синдромом старческой астении.
Fig. 1. Key pathological links determining the unfavorable outcome of COVID-19 in patients with senile asthenia syndrome.
ческой астенией, но и тех, у кого отсутствовали ее клинические проявления [79–82]. Нарушение структуры, сократительной способности, снижение выносливости грудных, дыхательных мышц и диафрагмы, уменьшение подвижности ребер негативно отражается на параметрах внешнего дыхания, вентиляции легких, увеличивает время больного на искусственной вентиляции легких, а также экстубирования и отлучения его от вентилятора становится причиной пролонгирования искусственной вентиляции легких, баротравмы легких, паралича, разрыва диафрагмы, стойкой икоты, вентиляторзависимой пневмонии, при дисфункции глотательных мышц — аспирационной пневмонии, что пагубно отражается на лече- нии острого респираторного дистресс-синдрома, исходе заболевания, особенно если у зараженных SARS-CoV-2 развилось осложнение в виде воспалительной или лекарственной миопатии критических состояний [83–93]. При аутопсии в диафрагме пациента с COVID-19, помимо экспрессии ангиотен-зинпревращающего фермента 2 (ACE2), удалось обнаружить и РНК SARS-CoV-2 [84, 94].
Поражение дыхательных мышц является важной независимой внелегочной первопричиной сохранения респираторных симптомов, периодического падения уровня сатурации при пульсоксиметрии в ближайшем и отдаленном периоде после инфицирования любым известным штаммом при наличии симптомокомплексов Post Intensive Care Syndrome (постреанимационная болезнь), post-acute COVID-19 syndrome, chronic COVID syndrome, Long COVID (постковидные синдромы) [95–99].
Апатия у пожилых людей во время пандемии развивается часто, но главным образом у паци- ентов с пограничными нервно-психическими расстройствами [100] и болезнью Альцгеймера [101]. По последним данным [102], вакцинация от коронавируса больным со старческой астенией во многих странах мира противопоказана: так, например, органы здравоохранения Норвегии, основываясь на печальном опыте высокой смертности, призывают перед вакцинацией проводить обязательное углубленное обследование всех пациентов в возрасте старше 65 лет на наличие у них симптомокомплекса старческой астении [103]. Целью скрининга является прогнозирование не только ответа на вакцинацию, но и заблаговременное предотвращение неблагоприятного исхода COVID-19. Кроме того, скрининг гарантирует проведение таргетной терапии у пациентов данной категории, позволяет отграничивать больных, нуждающихся в госпитализации, от тех, кому безопаснее и целесообразнее получить помощь и необходимый уход вне больничных учреждений [74, 104]. Для этой цели рекомендуется использовать шкалы EFS [105], The Hospital Frailty Risk Score (HFRS) [106], PRISMA-7 [107], FRAIL [108], CFS [109], расчет индекса «старческой хрупкости» (FI) [110]. Однако в большинстве зарубежных публикаций предпочтение отдается клинической шкале «старческой хрупкости» (CFS), преимуществами которой являются легкость применения, отсутствие необходимости в использовании дополнительных методов обследования [109].
Анализ научных статей, опубликованных в течение последних 6 мес, свидетельствует о большом интересе к изучению влияния синдрома старческой астении и ассоциированных с ним форм коморбидной патологии на исход заболевания при COVID-19. В ретроспективном когортном исследовании с участием пациентов в возрасте 59–85 лет с перенесенной коронавирусной инфекцией COVID-19 Z. Steinmeyer и соавт. [111] выявили, что 37% больных имели хронические респираторные заболевания, 11% — сахарный диабет, 69% — гипертоническую болезнь, 48% — другие сердечно-сосудистые заболевания, что объясняет наличие полипрагмазии в 44–65% случаев в зависимости от формы патологии. Средняя продолжительность пребывания в стационаре для большинства пациентов составляла 12 дней, из них у 10% диагностирована старческая астения, у 45% — деменция, у 30% — различные формы ограничения двигательной активности. Вынужденная длительная изоляция пациентов при COVID-19, негативное воздействие инфекции на головной мозг могут спровоцировать развитие делирия, усугубить его течение не только при наличии деменции, но и старческой астении.
ЗАКЛЮЧЕНИЕ
На основании изложенных данных можно сделать вывод, что старческая астения является одним из неблагоприятных исходов старения, но не неотъемлемой его частью.
Темп развития и полиморфизм клинических проявлений старческой астении определяются совокупностью генетических, психосоциальных, биологических и других факторов, негативно влияющих на человека на протяжении его жизни.
Лица, прожившие более 60 лет, склонны к развитию старческой астении и саркопении, но не у всех из них с диагностированной саркопенией
эактика
формируется симптомокомплекс, характерный для старческой астении.
Снижение физической активности, неполноценное питание, развитие торакального миостеатоза, саркопении, прогрессирующее нарушение функции грудных, дыхательных мышц и диафрагмы определяют неблагоприятное течение и исход у больных COVID-19 и старческой астенией.
ДОПОЛНИТЕЛЬНАЯ ИНФОРМАЦИЯ
Вклад авторов. Д.К. Веселова — анализ литературы, написание текста, подготовка иллюстраций; В.В. Белопасов — идея и концепция обзора, корректура текста. Авторы подтверждают соответствие своего авторства международным критериям ICMJE (все авторы внесли существенный вклад в разработку концепции, проведение исследования и подготовку статьи, прочли и одобрили финальную версию перед публикацией).
Author contribution. D.K. Veselova — literature analysis, manuscript editing, illustrations; V.V. Belo-pasov — the idea and concept of the review, text proofreading. The authors made a substantial contribution to the conception of the work, acquisition, analysis, interpretation of data for the work, drafting and revising the work, final approval of the version to be published and agree to be accountable for all aspects of the work.
Источник финансирования. Авторы заявляют об отсутствии внешнего финансирования при проведении поисково-аналитической работы.
Funding source. This study was not supported by any external sources of funding.
Список литературы Старческая астения и старческая апатия в повседневной клинической практике в условиях пандемии новой коронавирусной инфекции COVID-19
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31. doi: 10.1093/ageing/afy169
- Турушева А.В., Фролова Е.В., Дегриз Ж.М. Эволюция теории старческой астении // Вестник Северо-Западного государственного медицинского университета. 2017. T. 9, № 1. P. 117-124. [Turusheva AV, Frolova EV, Degriz JM. Evolution of the theory of senile asthenia. Bulletin of the North-Western State Medical University. 2017;9(1):117-124. (In Russ).]
- Wan H, Goodkind D, Kowal P. International Population Reports. P95/16-1. An Aging World: 2015 Washington. dC: u.S. Government Publishing Office; 2016. 165 р.
- Cardoso AL, Fernandes A, Aguilar-Pimentel JA, et al. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Aging Res Rev. 2018; 47:214-277. doi: 10.1016/j.arr.2018.07.004
- Woodhouse KW, Wynne H, Baillie S, James OF, Rawlins MD. Who are the frail elderly? Q J Med. 1988 Jul;68(255):505-6. doi: 10.1093/ OXFORDJOURNALS.QJMED.A068216
- Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8(1):1-17.
- Клинические рекомендации «Старческая астения». Москва, 2020. 88 с. [Clinical recommendations «Senile asthenia». Moscow; 2020. 88 p. (In Russ).]
- Щербакова Е.М. Старение населения мира по оценкам ООН 2019 года // Демоскоп Weekly. 2019. № 837-838. С. 1-26. [Shcherbakova EM. The aging of the world's population according to UN estimates in 2019. Demoscope Weekly. 2019;(837-838):1-26. (In Russ).]
- Faller JW, Pereira DN, de Souza S, et al. Instruments for the detection of frailty syndrome in older adults: a systematic review. PLOS One. 2019;14(4):55-64. doi: 10.1371/journal.pone.0216166
- Guidet B, de Lange DW, Boumendil A, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46(1):57-69. doi: 10.1007/s00134-019-05853-1
- Penteado CT, Loureiro JC, Pais MV, et al. Mental health status of psychogeriatric patients during the 2019 new coronavirus disease (COVID-19) pandemic and effects on caregiver burden. Front Psychiatry. 2020;11:578672. doi: 10.3389/fpsyt.2020.578672
- Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targetingand care. J Gerontol A Biol Sci Med Sci. 2004;59(3): 255-263. doi: 10.1093/gerona/glaa280
- Чукаева И.И., Ларина В.Н., Карпенко Д.Г., Ларин В.Г Новое направление в оценке приверженности лечению — акцент на гериатрические синдромы // Кардиоваскулярная терапия и профилактика. 2017. Т. 16, № 3. С. 46-51. [Chukaeva II, Larina VN, Karpenko DG, Larin VG. A new direction in assessing treatment adherence — emphasis on geriatric syndromes. Cardiovascular Therapy and Prevention. 2017;16(3):46-51. (In Russ).] doi: 10.15829/1728-8800-2017-3-46-51
- Carini G, Musazzi L, Bolzetta F, et al. The potential role of miRNAs in cognitive frailty. Front Aging Neurosci. 2021;13:763110. doi: 10.3389/fnagi.2021.763110
- He B, Chen W, Zeng J, et al. MicroRNA-326 decreases tau phos-phorrylation and neuron apoptosis through inhibition of the JNK signaling pathway by targeting VAV1 in Alzheimer's disease. J Cell Physiol. 2020;235:480-493. doi: 10.1002/jcp.28988
- Zhang Q, Wu X, Yang J. MiR-194-5p protects against myocardial ischemia/reper-fusion injury via MAPK1/PTEN/AKT pathway. Ann Transl Med. 2021;9:654. doi: 10.21037/atm-21-807
- Vatic M, von Haehling S, Ebner N. Inflammatory biomarkers of frailty. Exp Gerontol. 2020;133:110858. doi: 10.1016/j.exger.2020.110858
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World J. 2001;1: 323-336. doi: 10.1100/tsw.2001.58
- Ткачева О.Н., Котовская Ю.В., Рунихина Н.К., и др. Клинические рекомендации «Старческая астения» // Российский журнал гериатрической медицины. 2020. № 1. С. 11-46. [Tkacheva ON, Kotovskaya YuV, Runikhina NK, et al. Clinical recommendations «Senile asthenia». Russian Journal of Geriatric Medicine. 2020;(1):11-46. (In Russ).] doi: 10.37586/2686-8636-1 -2020-11-46
- Сафонова Ю.А., Зоткин Е.Г. Диагностическая значимость функциональных тестов для оценки возраст-ассоцииро-ванной саркопении // Остеопороз и остеопатии. 2016. Т. 19, № 2. С. 109-109. [Safonova YuA, Zotkin EG. Diagnostic significance of functional tests for the assessment of age-associated sarcopenia. Osteoporosis and Osteopathies. 2016;19(2):109-109. (In Russ).] doi: 10.14341/osteo20162109-109
- Сафонова Ю.А. Саркопения как фактор риска падений и переломов // Клиницист. 2019. Т. 13, № 3-4. С. 22-28. [Safonova YuA. Sarcopenia as a risk factor for falls and fractures. Clinician. 2019;13(3-4):22-28. (In Russ).]
- Клинические тесты в гериатрии. Методические рекомендации / под ред. проф. О.Н. Ткачевой. Москва: Прометей, 2019. 62 с. [Clinical tests in geriatrics. Methodological recommendations. Ed. by O.N. Tkacheva. Moscow: Prometey; 2019. 62 p. (In Russ).]
- Полищук Ю.И., Летников З.В. Синдром старческой астении в геронтологии и гериатрии с точки зрения герон-топсихиатрии // Социальная и клиническая психиатрия. 2018. Т. 28, № 4. С. 71-74. [Polishchuk YuI, Letnikova ZV. Senile asthenia syndrome in gerontology and geriatrics from the point of view of gerontopsychiatry. Social and Clinical Psychiatry. 2018;28(4):71-74. (In Russ).]
- Rosenberg PB, Lanctot KL, Drye LT, et al. ADMET Investigators. Safety and efficacy of methylphenidate for apathy in Alzheimer's disease: a randomized, placebo-controlled trial. J Clin Psychiatry. 2013;74(8):810-816.
- Turner G, Clegg A; British Geriatrics Society; Age UK; Royal College of General Practione. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43(6):744-747. doi: 10.1093/ageing/afu138
- García ML, Fernández A, Solas MT. Mitochondria, motor neurons and aging. J Neurol Sci. 2013;330(1-2):18-26. doi: 10.1016/j.jns.2013.03.019
- Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuro-muscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci. 2014;6:208. doi: 10.3389/fnagi.2014.00208
- Da Luz MC, Pinho CP, Bezerra GK, et al. SARC-F and SARC-CalF in screening for sarcopenia in older adults with Parkinson's disease. Exp Gerontol. 2021;144:111183. doi: 10.1016/j.exger.2020.111183
- Waite SJ, Maitland S, Thomas A, Yarnall AJ. Sarcopenia and frailty in individuals with dementia: a systematic review. Arch Gerontol Geriatr. 2021;92:104268. doi: 10.1016/j.archger.2020.10426
- Gómez-Gómez ME, Zapico SC. Frailty, cognitive decline, neurodegenerative diseases and nutrition interventions. Int J Mol Sci. 2019;20(11):2842. doi: 10.3390/ijms20112842
- Hofmeister F, Baber L, Ferrari U, et al. Late-onset neuromuscular disorders in the differential diagnosis of sarcopenia. BMC Neurol. 2021;21(1):241. doi: 10.1186/s12883-021-02264-y
- Королева М.В., Кудашкина Е.В, Шарова А.А., и др. Саркопения как предиктор снижения социализации и качества жизни у пациентов старшего возраста // Научные результаты биомедицинских исследований. 2019. Т. 5, № 4. С. 150-159. [Koroleva MV, Kudashkina EV, Sharova AA, et al. Sarcopenia as a predictor of decreased socialization and quality of life in older patients. Scientific Results of Biomedical Research. 2019;5(4): 150-159. (In Russ).]
- Гуляев Н.И., Ахметшин И.М., Гордиенко А.В., и др. Саркопения. Взгляд терапевта // Клиническая патофизиология. 2019. Т. 25, № 1. С. 3-8. [Gulyaev NI, Akhmetshin IM, Gordienko AV, et al. Sarcopenia. The therapist's view. Clinical Pathophysiology. 2019;25(1):3-8. (In Russ).]
- Сулейманова А.К., Сафонова Ю.А., Баранова И.А. Частота саркопении у пациентов со стабильной хронической об-структивной болезнью легких: сравнение диагностических алгоритмов Европейской рабочей группы по саркопении у пожилых людей (редакция 2010 и 2018 гг.) // Пульмонология. 2019. Т. 29, № 5. С. 564-570. [Suleymanova AK, Safonova YuA, Baranova IA. Frequency of sarcopenia in patients with stable chronic obstructive pulmonary disease: comparison of diagnostic algorithms of the European Working Group on Sarcopenia in the Elderly (revision 2010 and 2018). Pulmonology. 2019;29(5):564-570. (In Russ).] doi: 10.18093/0869-0189-2019-29-5-564-570
- Ахметшин И.М. Диагностическое значение саркопе-нии в оценке фильтрационной функции почек у больных хронической сердечной недостаточностью: Дис. ... канд. мед. наук. Санкт-Петербург, 2020. 149 с. [Akhmetshin IM. Diagnostic significance of sarcopenia in the assessment of filtration function of kidneys in patients with chronic heart failure [dissertation]. Saint Petersburg; 2020. 149 p. (In Russ).]
- Beeri MS, Leugrans SE, Delbono O, et al. Sarcopenia is associated with incident Alzheimer's dementia, mild cognitive impairment, and cognitive decline. J Am Geriatr Soc. 2021; 69(7):1826-1835. doi: 10.1111/jgs.17206
- Yuksel H, Balaban M, Tan OO, Mungan S. Sarcopenia in patients with multiple sclerosis. Mult Scler Relat Disord. 2021; 58:103471. doi: 10.1016/j.msard.2021.103471
- Мокрышева Н.Г., Крупинова Ю.А., Володичева В.Л., и др. Саркопения глазами эндокринолога // Ожирение и метаболизм. 2018. Т. 15, № 3. С. 21-27. [Mokrysheva NG, Krupinova YuA, Volodicheva VL, et al. Sarcopenia through the eyes of an endocrinologist. Obesity and Metabolism. 2018;15(3):21-27. (In Russ).] doi: 10.14341/OMET9792
- Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options — a mini-review. Gerontology 2014;60(4):294-305. doi: 10.1159/000356760
- Park CH, Do JG, Lee YT, Yoon KJ. Sarcopenic obesity associated with high-sensitivity C-reactive protein in age and sex comparison: a two-center study in South Korea. BMJ Open. 2018;8:e021232. doi: 10.1136/bmjopen-2017-021232
- Kaji H. Interaction between Muscle and Bone. J Bone Metab. 2014;21(1):29-40. doi: 10.11005/jbm.2014.21.1.29
- Correa-de-Araujo R, Addison O, Miljkovic I, et а!. Myosteatosis in the contxt of skeletal muscle function deficit: an interdisciplinary workshop at the national institute on aging. Front Physiol. 2020;11:963. doi: 10.3389/fphys.2020.00963
- Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med. 2016;31(6):1054-1060. doi: 10.3904/kjim.2016.193
- Сулейманова А.К. Синдром остеосаркопении у пациентов с хронической обструктивной болезнью легких: Автореф. дис. ... канд. мед. наук. Москва, 2020. 23 с. [Suleymanova AK. Osteosarcopenia syndrome in patients with chronic obstructive pulmonary disease [abstract dissertation]. Moscow; 2020. 23 p. (In Russ).]
- Тополянская С.В. Роль интерлейкина 6 при старении и возрастассоциированных заболеваниях // Клиницист. 2020. Т. 14, № 3-4. С. 10-17. [Topolyanskaya SV. The role of interleukin 6 in aging and age-associated diseases. Clinician. 2020;14(3-4):10-17. (In Russ).] doi: 10.17650/1818-8338-2020-14-3-4-K633
- Тополянская С.В. Саркопения, ожирение, остеопороз и старость // Сеченовский вестник. 2020. Т. 11, № 4. С. 23-35. [Topolyanskaya SV. Sarcopenia, obesity, osteoporosis and old age. Sechenovsky Bulletin. 2020;11(4):23-35. (In Russ).] doi: 10.47093/2218-7332.2020.11.4.23-35
- Григорьева И.И., Раскина Т.А., Летаева М.В., и др. Саркопения: особенности патогенеза и диагностики // Фундаментальная и клиническая медицина. 2019. Т. 4, № 4. С. 105-116. [Grigorieva II, Raskina TA, Letaeva MV, et al. Sarcopenia: features of pathogenesis and diagnosis. Fundamental and Clinical Medicine. 2019;4(4):105-116. (In Russ).] doi: 10.23946/2500-0764-2019-4-4-105-116
- Шостак Н.А., Мурадянц А.А., Кондратов А.А. Сарко-пения и перекрестные синдромы — значение в клинической практике // Клиницист. 2016. Т. 10, № 3. С. 10-14. [Shostak NA, Muradyants AA, Kondrashov AA. Sarcopenia and cross syndromes — significance in clinical practice. Clinician. 2016;10(3):10-14. (In Russ).] doi: 10.17650/1818-8338-2016-10-3-10-14
- Tagliafico AS, Bignotti B, Torri L, Rossi F. Sarcopenia: how to measure, when and why. Radiol Med. 2022. doi: 10.1007/s11547-022-01450-3
- Mohieldin S, Batsis JA, Minor CM, et al. Band Pass: a bluetooth-enabled remote monitoring device for sarcopenia. IEEE Int Conf Commun Workshops. 2021 ;2021:10.1109/iccworkshops50388.2021.9473520. doi: 10.1109/iccworkshops50388.2021.9473520
- Закревский А.И., Федорова А.А., Пасечник И.Н., Куте-пов Д.Е. Саркопения: как ее диагностировать? // Клиническое питание и метаболизм. 2021. Т. 2, № 1. С. 13-22. [Zakrevsky AI, Fedorova AA, Pasechnik IN, Kutepov DE. Sarcopenia: how to diagnose it? Clinical Nutrition and Metabolism. 0001;0(1):13-00. (In Russ).] doi: 10.178-16/clinutr71107
- Масенко В.Л., Коков А.Н., Григорьева И.И., Кривоша-пова К.Е. Лучевые методы диагностики саркопении // Исследования и практика в медицине. 2019. Т. 6, № 4. С. 127-137. [Masenko VL, Kokov AN, Grigorieva II, Krivoshapova KE. Radiation methods of diagnosis of sarcopenia. Research and Practice in Medicine. 2019;6(4):127-137. (In Russ).] doi: 10.17709/2409-2231-0019-6-4-13
- Lee K, Shin Y, Huh J, et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol. 0019;00(0): 205-217. doi: 10.3348/kjr.2018.0479
- Albano D, Messina C, Vitale J, Sconfienza LM. Imaging of sarcopenia: old evidence and new insights. Eur Radiol. 2020; 30(4):0199-0008. doi: 10.1007/s00330-019-06573-2
- Cohen E, Bay AA, Ni L, Hackney ME. Apathy-related symptoms appear early in parkinson's disease. Healthcare (Basel). 0000;10(1):91. doi: 10.3390/healthcare1001-0091
- Radakovic R, Colville S, Cranley D, et al. Multi-dimensional apathy in behavioral variant frontotemporal dementia, primary progressive aphasia, and Alzheimer disease. J Geriatr Psychiatry Neurol. 2021 ;34(5):349-356. doi: 10.1177/0891988720924716
- Jonsson M, Edman А, Lind K, et al. Apathy is a prominent neuropsychiatric feature of radiological white-matter changes in patients with dementia. Int J Geriatr Psychiatry. 2010;25(6):588-595. doi: 10.1002/gps.2379
- Аведисова А.С., Гехт А.Б., Захарова К.В., и др. Апатия в структуре психических и неврологических расстройств позднего возраста // Журнал неврологии и психиатрии им. С.С. Корсакова. 2014. Т. 114, № 6. С. 77-85. [Avedisova AS, Geht AB, Zakharova KV, et al. Apathy in the structure of mental and neurological disorders of late age. J Neurology and Psychiatry named after S.S. Korsakov. 2014;114(6):77-85. (In Russ).]
- Furneri G, Platania S, Privitera A, et al. The Apathy Evaluation Scale (AES-C): psychometric properties and invariance of Italian version in mild cognitive impairment and Alzheimer's disease. Int J Environ Res Public Health. 2021;18(18):89597. doi: 10.3390/ijerph18189597
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16(7):916-928. doi: 10.1093/cercor/bhj043
- Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. Eur Psychiatry. 0009;04(0):98-104. doi: 10.1016/j.eurpsy.2008.09.001
- Guercio BJ, Donovan NJ, Munro CE, et al. The apathy evaluation scale: a comparison of subject, informant, and clinician report in cognitively normal elderly and mild cognitive impairment. J Alzheimers Dis. 0015;47(0):401-430. doi: 10.3233/JAD-150146
- Robert P, Lanctôt KL, Agüera-Ortiz L, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry. 2018;71-76. doi: 10.1016/j.eurpsy.2018.07.008
- Золотарева А.А. Теоретический анализ проблемы диагностики апатии // Клиническая и специальная психология. 2021. Т. 10, № 3. С. 17-30. [Zolotareva AA. Theoretical analysis of the problem of apathy diagnosis. Clinical and Special Psychology. 2021;10(3):17-30. (In Russ).] doi: 10.17759/cpse.0001100300
- Прокопенко С.В., Баранкин Б.В., Марьина Н.М., и др. Клинический случай применения ПЭТ/КТ в ранней диагностике болезни Альцгеймера // Анналы клинической и экспериментальной неврологии. 2017. Т. 11, № 4. С. 65-70. [Prokopenko SV, Barankin BV, Maryina NM, et al. Clinical case of PET application/CT in the early diagnosis of Alzheimer's disease. Annals of Clinical and Experimental Neurology. 2017;11(4):65-70. (In Russ).] doi: 10.18454/ACEN.2017.4.7
- Jonsson M, Edman А, Lind K, et al. Apathy is a prominent neuropsychiatric feature of radiological white-matter changes in patients with dementia. J Clin Exp Neuropsychol. 2010;25(6): 588-595. doi: 10.1002/gps.2379.65
- Le Heron C, Apps MA, Husain M. The anatomy of apathy: a neurocognitive framework for amotivated behaviour. Neuropsychologia. 2018;118(PtB):54-67. doi: 10.1016/j.neuropsycho-logia.2017.07.003
- Chilovi BV, Rozzini L, Bertoletti E, et al. Angiotensin converting enzyme (ACE) inhibitors modulate the rate of progression of amnestic mild cognitive impairment. Int J Geriatr Psychiatry 2006;21(6):550-555. doi: 10.1002/gps.1523
- Ruthirakuhan M, Herrmann N, Vieira D, et al. The roles of apathy and depression in predicting Alzheimer disease: a longitudinal analysis in older adults with mild cognitive impairment. Am J Geriatr Psychiatry. 2019;27(8):873-882. doi: 10.1016/j.jagp.0019.00.003
- Ma L. Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Front Aging Neurosci. 0000;10:9. doi: 10.3389/fnagi.2020.00009
- Roberto N, Portella MJ, Marquié M, et al. Neuropsychiatric profiles and conversion to dementia in mild cognitive impairment, a latent class analysis. Sci Rep. 2021;11(1):6448. doi: 10.1038/s41598-021 -83126-y
- Федулкина В.А., Ватазин А.В., Кильдюшевский А.В., и др. Иммуносенесценция как причина индивидуализированной иммуносупрессивной терапии при трансплантации почки // Вестник трансплантологии и искусственных органов. 2021. Т. 23, № 3. С. 171-179. [Fedulkina VA, Vatazin AV, Kildyushevsky AV, et al. Immunosenescence as a cause of individualized immuno-suppressive therapy in kidney transplantation. Bulletin of Transplantology and artificial Organs. 0001;03(3):171-179. (In Russ).] doi: 10.15825/1995-1191-2021-3-171-179
- Martins PN, Tullius SG, Markmann JF. Immunosenescence and immune response in organ transplantation. Int Rev Immunol. 2014;33(3):162-173. doi: 10.3109/08830185.2013.829469
- Ali AM, Kunugi H. Screening for sarcopenia (physical frailty) in the COVID-19 era. Int J Endocrinol. 0001;0001:5563960. doi: 10.1155/2021/5563960
- Hussien H, Nastasa A, Apetrii M, et al. Different aspects of frailty and COVID-19: points to consider in the current pandemic and future ones. BMC Geriatrics. 0001;01(1):389. doi: 10.1186^12877-021-02316-5
- Lengelé L, Locquet M, Moutschen M, et al. Frailty but not sarcopenia nor malnutrition increases the risk of developing COVID-19 in older community-dwelling adults. Aging Clin Exp Res. 0000;34(1):003-034. doi: 10.1007/s40520-021-01991-z
- Kundi H, Çetin EH, Canpolat U, et al. The role of frailty on adverse outcomes among older patients with COVID-19. J Infect. 2020;81 (6):944-951. doi: 10.1016/j.jinf.0000.09.009
- Pilotto A, Veronese N, Siri G, et al. Association between the multidimensional prognostic index and mortality over 15 years of follow-up in the inchianti study. J Gerontol A Biol Sci Med Sci. 2020;76:1678-1685. doi: 10.1093/gerona/glaa237
- Morley J, Kalantar-Zadeh K, Anker S. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11 (4):863-865. doi: 10.1002^^1.12589
- Meftahi G, Jangravi Z, Sahraei H, Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of "inflame-aging". Inflamm Res. 0000;69(9):805-839. doi: 10.1007^00011-020-01372-8
- Mittal A, Dua A, Gupta S, Injeti E. A research update: significance of cytokine storm and diaphragm in COVID-19. Curr Res Pharmacol Drug Discov. 0001;0:100031. doi: 10.1016/j.crphar.0001.100031
- Yi X, Liu H, Zhu L, et al. Myosteatosis predicting risk of transition to severe COVID-19 infection. Clin Nutr. 2021; S0061-5614(01)00081-8. doi: 10.1016^x^.2021.05.031
- Ohara D, Pegorari M, Oliveira Dos Santos N, et al. Cross-sectional study on the association between pulmonary function and sarcopenia in brazilian community-dwelling elderly from the amazon region. J Nutr Health Aging. 0000;04(0):181-187. doi: 10.1007/s12603-019-1290-y
- Shi Z, de Vries HJ, Vlaar AP, et al.; Dutch COVID-19 Diaphragm Investigators. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med. 2021;181(1):122-124. doi: 10.1001/jamainternmed.2020.6278
- Antonarelli M, Fogante M. Chest CT-derived muscle analysis in COVID-19 patients. Tomography. 2022;8(1):414-422. doi: 10.3390/tomography8010034
- Chianca V, Albano D, Messina C, et al. Sarcopenia: imaging assessment and clinical application. Abdom Radiol (NY). 2021;1-12. doi: 10.1007/s00261 -021-03294-3
- Schiaffino S, Albano D, Cozzi A, et al. CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology. 2021;300(2):E328-E336. doi: 10.1148/radiol.2021204141
- Loosen SH, Schulze-Hagen M, Püngel T, et al. Skeletal muscle composition predicts outcome in critically ill patients. Crit Care Explor. 2020;2(8):e0171. doi: 10.1097/CCE.0000000000000171
- Corradi F, Isirdi A, Malacarne P, et al.; UCARE (Ultrasound in Critical care and Anesthesia Research Group). Low diaph-ragm muscle mass predicts adverse outcome in patients hospitalized for COVID-19 pneumonia: an exploratory pilot study. Minerva Anestesiol. 2021;87(4):432-438. doi: 10.23736/S0375-9393.21.15129-6
- FitzMaurice TS, McCann C, Walshaw M, Greenwood J. Unilateral diaphragm paralysis with COVID-19 infection. BMJ Case Rep. 2021;14(6):e243115. doi: 10.1136/bcr-2021-243115
- Nasir S, Shahid O, Nasir SA, Khan MW. Unilateral diaphragmatic paralysis in a patient with COVID-19 pneumonia. Ali Cureus. 2021;13(11):e19322. doi: 10.7759/cureus.19322
- Poggiali E, Vercelli A, Demichele E, et al. Diaphragmatic rupture and gastric perforation in a patient with COVID-19 pneumonia. Eur J Case Rep Intern Med. 2020;7(6):001738. doi: 10.12890/2020_001738
- Atiyat R, Veeraballi S, Al-Atiyat N, et al. Rare case report of persistent hiccups as an atypical presentation of COVID-19. Cureus. 2021;13(3):e13625. doi: 10.7759/cureus.13625
- Patel Z, Franz CK, Bharat A, et al. Diaphragm and phrenic nerve ultrasound in covid-19 patients and beyond: imaging technique, findings, and clinical applications. J Ultrasound Med. 2022;41(2): 285-299. doi: 10.1002/jum.15706
- Белопасов В.В., Журавлева Е.Н., Нугманова Н.П., Абдрашитова А.Т. Постковидные неврологические синдромы // Клиническая практика. 2021. Т. 12, № 2. С. 69-82. [Belopasov VV, Zhuravleva EN, Nugmanova NP, Abdrashitova AT. Postcovid neurological syndromes. Journal of Clinical Practice. 2021;12(2): 69-82. (In Russ).] doi: 10.17816/clin-pract71137
- Soares MN, Eggelbusch M, Naddaf E, et al. Skeletal muscle alterations in patients with acute COVID-19 and post-acute sequelae of COVID-19. J Cachexia Sarcopenia Muscle. 2022;13(1):11-22. doi: 10.1002/jcsm.12896
- Farr E, Wolfe AR, Deshmukh S, et al. Diaphragm dysfunction in severe COVID-19 as determined by neuromuscular ultrasound. Ann Clin TranslNeurol. 2021;8(8):1745-1749. doi: 10.1002/acn3.51416
- Kardas H, Thormann M, Bär C, et al. Impact of pectoral muscle values on clinical outcomes in patients with severe COVID-19 disease. In Vivo. 2022;36(1):375-380. doi: 10.21873/invivo.12713
- Carfl A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603-605.
- Gracia-García P, Modrego P, Lobo A. Apathy and neurocognitive correlates: review from the perspective of 'precision psychiatry'. Curr Opin Psychiatry. 2021;34(2):193-198. doi: 10.1097/YC0.0000000000000677
- Lara B, Carnes A, Dakterzada F, et al. Neuropsychiatric symptoms and quality of life in Spanish patients with Alzheimer's disease during the COVID-19 lockdown. Eur J Neurol. 2020; 27(9): 1744-1747. doi: 10.1111/ene.14339
- Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAd0x1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979-1993. doi: 10.1016/S0140-6736(20)32466-1
- Torjesen I. Covid-19: doctors in Norway told to assess severely frail patients for vaccination. BMJ. 2021;372:n167. doi: 10.1136/bmj.n167
- Ensrud KE, Kats AM, Schousboe JT, et al. Frailty phenotype and healthcare costs and utilization in older women. J Am Geriatr Soc. 2018;66(7):1276-1283. doi: 10.1111/jgs.15381
- Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton frail scale. Age Ageing. 2006;35(5): 526-529. doi: 10.1093/ageing/afl041
- Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/s0140-6736(18)30668-8
- O'Caoimh R, Costello M, Small C, et al. Comparison of frailty screening instruments in the emergency department. Int J Environ Res Public Health. 2019;16(19):3626. doi: 10.3390/ijerph16193626
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601-608. doi: 10.1007/s12603-012-0084-2
- Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23(9):771-787. doi: 10.1007/s12603-019-1273-z
- Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. doi: 10.1186/1471-2318-8-24
- Steinmeyer Z, Vienne-Noyes S, Piau A, et al. Acute care of older patients with COVID-19: clinical characteristics and outcomes. Geriatrics. 2020;5(4):65. doi: 10.3390/geriatrics5040065


