Synthesis and structure of µ2-oxo-bis [(pentafluoropropionato)-tris(5-bromo-2-methoxyphenyl)antimony]
Автор: Artemeva E.V.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 3 т.11, 2019 года.
Бесплатный доступ
The synthesis of μ2-oxo- bis [(pentafluoropropionato) tris (5-bromo-2-methoxyphenyl)antimony] (1) has been curried out by the oxidative addition reaction of tris (5-bromo-2-methoxyphenyl)antimony with pentafluoropropionic acid in the presence of hydrogen peroxide at an equimolar ratio of reactants. The compound is characterized by IR spectroscopy and X-ray diffraction analysis. According to X-ray diffraction data, there are two types of crystallographically independent molecules with slightly different geometrical parameters in the crystal. The antimony atoms in each molecule are linked by the bridging oxygen atom (the SbOSb angle is 167.6(7)°). The trigonal bipyramidal coordination of antimony atoms is distorted; the axial OSbO angles are 175.9(4)°, 174.5(4)° for Sb(1) and Sb(2) atoms, respectively. The sums of valence CSbC angles in the equatorial planes are 359.6(6)° and 356.4(6)°. The mean values of the Sb(1)-C and Sb(2)-C bond lengths are 2.12(2) Å, the Sb-O( μ 2) distances are 1.89(1), 1.98(1) Å. The carboxylate ligands in each molecule are monodentate. The distances between the antimony atoms and the corresponding terminal oxygen atoms are 2.22(1), 2.39(1) Å, the Sb···O=C distances are 3.38(2), 3.71(1) Å. The d(Sb···O=C)/d(Sb-О) ratios, which can be used to estimate the asymmetry of the ligand related to metal atom coordination, are 1.67 and 1.41. In crystal 1 there are such contacts as Sb···OMe (2.88(1)-3.16(1), 3.01(1)-3.20(1) Å), hydrogen bonds involving halogen atoms H···F (2.16, 2.50, 2.58 Å), H···Br (2.92, 2.96, 3.04 Å) and carbonyl oxygen atoms H···О (2.43, 2.59 Å), as well as halogen···halogen F···F interactions (2.70, 2.71 Å).
Tris(5-bromo-2-methoxyphenyl)antimony, pentafluoropropionic acid, oxidative addition, structure, x-ray diffraction analysis, ir spectroscopy
Короткий адрес: https://sciup.org/147233139
IDR: 147233139 | УДК: 546.865+547.1-32+547.304.6+547.53.024+548.312.5 | DOI: 10.14529/chem190310
Текст научной статьи Synthesis and structure of µ2-oxo-bis [(pentafluoropropionato)-tris(5-bromo-2-methoxyphenyl)antimony]
It is known that triarylantimony complexes with carboxylate ligands are biologically active compounds, having antibacterial [1, 2], antileishmanial [2–5], photoluminescent [6] properties; they act as photocatalysts [7] and reagents in organic synthesis [8]. Various triarylantimony dicarboxylates Ar3SbX2 were obtained by substitution [1–3, 5–8] and oxidative addition reactions, with the molar ratio of triaryl antimony and carboxylic acid 1:2 [4, 9–11]. Synthesis of binuclear triarylantimony carboxylates with monodentate ligands has been described in a few papers only [12–17]. It is obvious that such compounds have not been studied enough, and a further study of the oxidative addition reactions of triarylantimony with carboxylic acids with an equimolar ratio of reagents, as well as the structure determination of the products obtained is of interest.
The present work is related to the study of the interaction of tris (5-bromo-2-methoxyphenyl)antimony with pentafluoropropionic acid in the presence of hydrogen peroxide at 1:1:1 molar ratio of the reactants and the structure determination of the reaction product.
Experimental
Synthesis of µ 2 -oxo- bis [(pentafluoropropionato) tris (5-bromo-2-methoxyphenyl)antimony] (1) Tris (5-bromo-2-methoxyphenyl)antimony (0.2 g, 0.29 mmol) and pentafluoropropionic acid (0.048 g, 0.29 mmol) were dissolved in 10 ml of diethyl ether, then 30 % aqueous solution of hydrogen peroxide (0.033 g, 0.29 mmol) was added. The mixture was kept for 24 h at 20 °C. 0.221 g (89 %) of colorless crystals of 1 with MP 203 °C was obtained.
IR spectrum, ν, сm–1: 1719, 1701, 1576, 1476, 1439, 1376, 1314, 1283, 1254, 1207, 1169, 1151, 1092, 1053, 1024, 881, 864, 808, 727, 708, 676, 619, 538, 521, 440, 424.
Found, %: С 33.85, Н 2.16. For C 38 H 31 Br 3 N 4 O 9 Sb calculated, %: С 33.88, Н 2.13.
Химия элементоорганических соединений
IR spectra of compound 1 were recorded on a Shimadzu IRAffinity-1S FTIR-spectrometer; samples were prepared by pelletting with KBr (absorption region 4000–400 cm–1).
X-ray diffraction analysis of crystalline substance 1 was performed on a Bruker D8 QUEST automatic four-circle diffractometer (Mo K α -emission, λ 0.71073 Å, graphite monochromator).
Data collection and editing, unit-cell parameters refinement, and correction for absorption were carried out in SMART and SAINT-Plus software [18]. All calculations aimed at solving and refining the structure of compound 1 were performed in SHELXL/PC software [19]. Structure 1 was determined by direct methods and refined with the least squares method in the anisotropic approximation for nonhydrogen atoms. Selected bond lengths and bond angles of 1 are summarized in Table 1.
Crystal Data for C 96 H 72 O 22 F 20 Br 12 Sb 4 ( M =3403.46 g/mol): triclinic, space group Pī, a 13.156(7) Å, b 17.932(11) Å, c 27.102(12) Å, α 90.05(3)°, β 90.02(2)°, γ 96.75(3)°, V 6349(6) Å3, Z 2, μ Mo 4.705 mm–1, D calc 1.780 g/cm3, 130469 reflections measured, 25008 unique reflections ( R int 0.1675), the number of refinement variables 1399, GOOF 1.016, R factors for F 2 > 2 σ ( F 2): R 1 0.1012, wR 2 0.2748, R factors for all reflections R 1 0.1820, wR 2 0.3267.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1912122 for compound 1; ; .
Table 1
Selected bond lengths and bond angles in structure 1
|
a |
|||||
|
Bond |
d , Å |
Angle |
ω , deg |
Angle |
ω , deg |
|
Sb(1)–С(1) |
2.12(1) |
Sb(1)O(6)Sb(2) |
167.6(7) |
O(1)Sb(1)C(1) |
89.6(5) |
|
Sb(1)–С(11) |
2.08(2) |
O(6)Sb(1)O(1) |
175.9(4) |
O(1)Sb(1)C(11) |
101.2(5) |
|
Sb(1)–С(21) |
2.17(1) |
O(6)Sb(2)O(10) |
174.5(4) |
O(10)Sb(2)C(31) |
98.4(5) |
|
Sb(2)–C(31) |
2.11(2) |
C(1)Sb(1)C(11) |
117.0(6) |
O(10)Sb(2)C(41) |
73.8(5) |
|
Sb(2)–C(41) |
2.15(1) |
C(11)Sb(1)C(21) |
118.2(6) |
O(10)Sb(2)C(51) |
78.4(5) |
|
Sb(2)–C(51) |
2.11(2) |
C(1)Sb(1)C(21) |
124.4(6) |
O(6)Sb(1)C(1) |
87.2(5) |
|
Sb(1)–O(1) |
2.22(1) |
C(31)Sb(2)C(51) |
126.1(6) |
O(6)Sb(1)C(11) |
82.5(6) |
|
Sb(1)–O(6) |
1.98(1) |
C(41)Sb(2)C(31) |
113.8(6) |
O(6)Sb(1)C(21) |
105.4(5) |
|
Sb(2)–O(10) |
2.39(1) |
C(41)Sb(2)C(51) |
116.5(6) |
O(6)Sb(2)C(41) |
101.7(5) |
|
Sb(2)–O(6) |
1.89(1) |
O(1)Sb(1)C(21) |
74.5(5) |
O(6)Sb(2)C(51) |
101.2(5) |
|
b |
|||||
|
Sb(3)–C(61) |
2.08(2) |
Sb(3)O(17)Sb(4) |
167.8(7) |
O(12)Sb(3)C(71) |
89.2(5) |
|
Sb(3)–C(71) |
2.13(1) |
O(12)Sb(3)O(17) |
175.9(4) |
O(12)Sb(3)C(81) |
74.8(5) |
|
Sb(3)–C(81) |
2.16(1) |
O(17)Sb(4)O(21) |
175.2(4) |
O(21)Sb(4)C(91) |
98.1(5) |
|
Sb(4)–C(91) |
2.11(1) |
C(61)Sb(3)C(71) |
115.7(6) |
O(21)Sb(4)C(101) |
78.4(5) |
|
Sb(4)–C(101) |
2.12(2) |
C(61)Sb(3)C(81) |
119.8(6) |
O(21)Sb(4)C(111) |
73.4(5) |
|
Sb(4)–C(111) |
2.16(2) |
C(71)Sb(3)C(81) |
124.1(5) |
O(17)Sb(3)C(61) |
82.6(6) |
|
Sb(3)–O(12) |
2.21(1) |
C(91)Sb(4)C(101) |
125.2(6) |
O(17)Sb(3)C(71) |
87.6(5) |
|
Sb(3)–O(17) |
1.98(1) |
C(91)Sb(4)C(111) |
114.1(6) |
O(17)Sb(3)C(81) |
104.9(5) |
|
Sb(4)–O(21) |
2.38(1) |
C(101)Sb(4)C(111) |
116.7(6) |
O(17)Sb(4)C(91) |
85.8(6) |
|
Sb(4)–O17) |
1.89(1) |
O(12)Sb(3)C(61) |
101.1(6) |
O(17)Sb(4)C(111) |
102.5(5) |
Results and Discussion
It has been found that the oxidative addition reaction of tris (5-bromo-2-methoxyphenyl)antimony with pentafluoropropionic acid in the presence of hydrogen peroxide at 1:1:1 molar ratio with the formation of binuclear organic antimony compound 1 with μ 2 -bridging oxygen atom:
2 (5-Br-2-MeOС 6 Н 3 ) 3 Sb + 2 CF 3 CF 2 C(O)OH +2 H 2 O 2 →
→ [(5-Br-2-MeOС 6 Н 3 ) 3 SbO(O)CCF 2 CF 3 ] 2 O + 2 H 2 O
Compound 1 is a crystalline substance, highly soluble in aromatic and aliphatic hydrocarbons, resistant to moisture and air oxygen.
Structure 1 has been determined by X-ray diffraction analysis and confirmed by IR spectroscopy.
In the IR spectrum of compound 1 there are bands at 521 cm–1, characterizing the Sb–C vibrations [20], and at 440 cm–1 due to the Sb–O vibrations [21]. The intense absorption band at 1719 cm–1 characterizes the carbonyl group C=O stretching vibrations, the band at 1375 cm–1 does it for the C–F bonds. The absorption bands at 1169 cm–1 and 1283 cm–1 correspond to vibrations of the CAr–Br and C Ar –OMe bonds, respectively.
According to X-ray diffraction data, in crystal 1 there are two types of crystallographically independent molecules a and b , the geometric parameters of which are equal within the error limits, therefore, in the following, we discuss the structural data of molecule 1 a . The antimony atoms in 1 have a distorted trigonal-bipyramidal coordination with oxygen atoms in axial positions (Fig. 1). The antimony atoms in the binuclear molecule are linked by the bridging oxygen atom, the Sb(1)O(6)Sb(2) bond angle is 167.6(7)°. The sums of the CSb(1)C and СSb(2)C valence angles in the equatorial planes are 359.6(6)° and 356.4(6)°, respectively, while the values of the individual angles differ from the theoretical 120° by no more than 6.2(6)º. The Sb(1,2) atoms deviate from the corresponding equatorial planes by 0.071 and 0.233 Å to the direction of the bridging oxygen atom. The axial OSb(1,2)O angles are 175.9(4)°, 174.5(4)°. The O term Sb(1,2)C angles vary within the ranges 74.5(5)º–101.2(5)º, 73.8(5)º– 98.4(5)º, O( μ 2 )Sb(1,2)C – 82.5(6)º–105.4(5)º, 86.3(6)º–101.7(5)º.
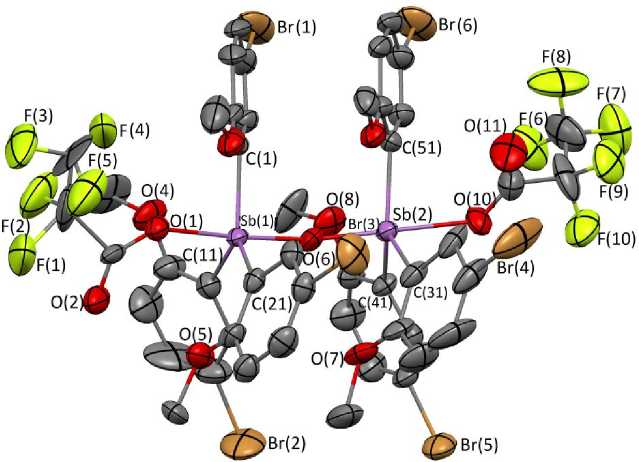
Fig. 1. Structure 1 a showing thermal ellipsoids at 30% probability.
Hydrogen atoms have been omitted for clarity
The average values of the Sb(1)–C and Sb(2)–C bond lengths are 2.12(2) Å. The Sb(1,2)–O( μ 2 ) distances (1.98(1), 1.89(1) Å) are less than Sb(1,2)–O term (2.22(1), 2.39(1) Å) and less than the sum of covalent radii of the antimony and oxygen atoms 2.07 Å [22].
Carboxylate ligands show anisobidentate properties, coordinated by a carbonyl oxygen atom to the antimony atom, the Sb(1,2)···O=C distances are 3.71 (1), 3.38 (2) Å. The coordination asymmetry of ligands can be estimated by the d (Sb···O=C)/ d (Sb–О term ) ratio, which is equal to 1.67 and 1.41.
In the molecule there are contacts Sb(1,2)···OMe, the corresponding distances equal 2.88(1)– 3.16(1), 3.01(1)–3.20(1) Å.
Molecules a and b in crystal 1 are linked by intermolecular contacts F···H Ar (2.50, 2.58 Å), F···H Me (2.16 Å). The structural organization of 1 is due to hydrogen bonds, involving oxygen atoms of carboxylate ligands H Ar ···O(=C) (2.43, 2.59 Å) and bromine atoms H Me ···Br (2.92, 2.96, 3.04 Å), as well as F···F contacts (2.70, 2.71 Å) of the first type (according to the classification given in [23]), θ 1 ,CF(7)F(3) (124(2)º) ≈ θ 2 ,CF(3)F(7) (125(4)º), θ 1 , CF(12)F(17) (116(4)º) ≈ θ 2 ,CF(17)F(12) (117(2)º) (Fig. 2).
Химия элементоорганических соединений
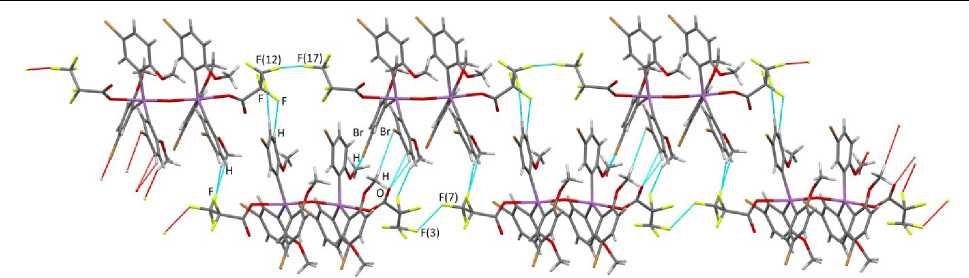
Fig. 2. The intermolecular interactions in 1
Conclusion
The oxidative addition reaction of tris(5-bromo-2-methoxyphenyl)antimony with pentafluoropropionic acid at 1:1 molar ratio in the presence of hydrogen peroxide leads to formation of the binuclear compound with a bridging oxygen atom, the structural organization of which is due to hydrogen bonds and contacts with halogen atoms.
Acknowledgments
We are grateful to V.V. Sharutin for the X-ray diffraction analysis of 1 and O.K. Sharutina for the article preparation to submission.
Список литературы Synthesis and structure of µ2-oxo-bis [(pentafluoropropionato)-tris(5-bromo-2-methoxyphenyl)antimony]
- Abdolmaleki S., Yarmohammadi N., Adibi H., Ghadermazi M., Ashengroph M., Rudbari H.A., Bruno G. Synthesis, X-ray Studies, Electrochemical Properties, Evaluation as in Vitro Cytotoxic and Antibacterial Agents of Two Antimony(III) Complexes with Dipicolinic Acid. Polyhedron, 2019, vol. 159, pp. 239-250. DOI: 10.1016/j.poly.2018.11.063
- Mushtaq R., Rauf M.K., Bond M., Badshah A., Ali M.I., Nadhman A., Yasinzai M., Tahir M.N. A Structural Investigation of Heteroleptic Pentavalent Antimonials and Their Leishmanicidal Activity. Appl. Organomet. Chem., 2016, vol. 30, no. 6, pp. 465-472. DOI: 10.1002/aoc.3456
- Saleem L., Altaf A.A., Badshah A., Rauf M.K., Waseem A., Danish M., Azam S.S., Arshad M.N., Asiri A.M., Ahmad S., Gul R. Structural Investigations, Anti-leishmanial, Antibacterial and Docking Studies of New Pentavalent Antimony Carboxylates. Inorg. Chim. Acta, 2018, vol. 474, pp. 148-155. DOI: 10.1016/j.ica.2018.01.036
- Duffin R.N., Blair V.L., Kedzierski L., Andrews P.C. Comparative Stability, Toxicity and Antileishmanial Activity of Triphenyl Antimony(V) and Bismuth(V) α-Hydroxy Carboxylato Complexes. Dalton Trans., 2018, vol. 47, pp. 971-980. DOI: 10.1039/c7dt04171c
- Keogan D.M., Oliveira S.S.C., Sangenito L.S., Branquinha M.H., Jagoo R.D., Twamley B., Santos A.L.S., Griffith D.M. Novel Antimony(III) Hydroxamic Acid Complexes as Potential Anti-Leishmanial Agents. Dalt. Trans., 2018, vol. 47, no. 21, pp. 7245-7255. DOI: 10.1039/c8dt00546j
- Qi H.-X., Jo H., Lee H.E., Hong J., Ok K.M. Crystals Of Sb3+-Coordination Complexes Exhibiting Yellowish Green Emissions With Outstanding Lifetimes. J. Solid State Chem., 2019, vol. 274, pp. 69-74.
- DOI: 10.1016/j.jssc.2019.03.018
- Zhang X.-Y., Cui L., Zhang X., Jin F., Fan Y.-H. Two Organoantimony (V) Coordination Complexes Modulated by Isomers of Trifluoromethylbenzoate Ligands: Syntheses, Crystal Structure, Photodegradation Properties. J. Mol. Struct., 2017, vol. 1134, pp. 742-750.
- DOI: 10.1016/j.molstruc.2017.01.039
- Qin W., Yasuike S., Kakusawa N., Sugawara Y., Kawahata M., Yamaguchi K., Kurita J. Triarylantimony Dicarboxylates as Pseudo-halides for Palladium-catalyzed Cross-coupling Reaction with Arylboronic Acids and Triarylbismuthanes without any base. J. Organomet. Chem., 2008, vol. 693, no. 1, pp. 109-116.
- DOI: 10.1016/j.jorganchem.2007.10.030
- Sharutin V.V., Senchurin V.S., Sharutina O.K., Kazakov M.V. Reactions of Tri-p-Tolylantimony with Carboxylic and Arylsulfonic Acids and Phenols. Russ. J. Gen. Chem., 2012, vol. 82, no. 1, pp. 95-98.
- DOI: 10.1134/S1070363212010161
- Sharutin V.V., Sharutina O.K., Reshetnikova R.V., Lobanova E.V., Efremov A.N. Tris(3-fluorophenyl)antimony Dicarboxylates (3-FC6H4)3Sb[OC(O)R]2 (R = CH2Cl, Ph, CH2C6H4NO2-4, C10H15): Synthesis and Structure. Rus. J. Inorg. Chem., 2017, vol. 62, no. 11, pp. 1450-1457.
- DOI: 10.1134/S003602361711016X
- Sharutin V.V., Sharutina O.K., Efremov A.N. Tri-Para-Tolylantimony Dicarboxylates (4-MeC6H4)3Sb[OC(O)R)]2, R = C6H4(NO2-3), C6H3(NO2)2-3,5, CH2Br: Synthesis and Structure. Russ. J. Inorg. Chem., 2019, vol. 64, no. 1, pp. 68-73.
- DOI: 10.1134/S0036023619010194
- Gibbons M.N., Sowerby D.B. Reactions of [SbR3X2]2O with Carboxylates and the Crystal Structures of [SbPh3(O2CCF3)2]2O and [SbMe3(O2CCH3)2]2O. J. Organomet. Chem., 1998, vol. 555, no. 2, pp. 271-278.
- DOI: 10.1016/S0022-328X(97)00759-6
- Quan L., Yin H., Wang D. μ-Oxido-bis[(chloroacetato-κO)triphenylantimony(V)]. Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, vol. 64Е. m349.
- DOI: 10.1107/S1600536808000676
- Quan L., Yin H., Wang D. μ-Oxido-bis[(2-chloronicotinato-κO)triphenylantimony (V)]. Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, vol. 65Е. m99.
- DOI: 10.1107/S1600536808042335
- Sharutin V.V., Sharutina O.K., Senchurin V.S. Tri- and Tetraphenylantimony Propiolates: Synthesis and Structures. Rus. J. of Coord. Chem., 2014, vol. 40, no. 2, pp. 109-114.
- DOI: 10.1134/S1070328414020109
- Sharutin V.V., Pakusina A.P., Sharutina O.K., Nasonova N.V., Gerasimenko A.V., Pushilin M.A. [Synthesis, Structure and the Reactions of Antimony Compounds]. Butlerovskie soobshhenija [Butlerov communications], 2002, no. 11. pp. 13-22. (in Russ.)
- Sharutin V.V., Sharutina O.K., Efremov A.N., Artem’eva E.V. Synthesis and Structure of μ2-Oxobis(carboxylatotriarylantimony). Russ. J. Gen. Chem., 2019, vol. 89, no. 1, pp. 76-81.
- DOI: 10.1134/s1070363219010146
- Bruker (2000) SMART. Bruker Molecular Analysis Research Tool, Versions 5.625 Bruker AXS, Madison, Wisconsin, USA.
- Bruker (2000) SAINTPlus Data Reduction and Correction Program, Versions 6.02a, Bruker AXS, Madison, Wisconsin, USA.
- Doak G.O., Long G.G., Freedman L.D. The Infrared Spectra of Some Phenylsubstituted Pentavalent Antimony Compounds. J. Organomet. Chem., 1965, vol. 4, no. 1, pp. 82-91.
- Gupta A., Sharma R.K., Bohra R., Jain V.K., Drake J.E., Hursthouse M.B., Light M.E. Synthetic, Spectroscopic and Structural Aspects of Triphenylantimony(V) Complexes with Internally Functionalized Oximes: Crystal and Molecular Structure of [Ph3Sb{ONC(Me)C5H4N-2}2]. Polyhedron, 2002, vol. 21, no. 23, pp. 2387-2392.
- DOI: 10.1016/S0277-5387(02)01155-5
- Batsanov S.S. [Atomic Radii of the Elements]. Rus. J. Inorg. Chem., 1991, vol. 36, no. 12, pp. 3015-3037. (in Russ.)
- Tothadi S., Joseph S., Desiraju G.R. Synthon Modularity in Cocrystals of 4-Bromobenzamide with n-Alkanedicarboxylic Acids: Type I and Type II Halogen···Halogen Interactions. Cryst. Growth Des., 2013, vol. 13, no. 7, pp. 3242-3254.
- DOI: 10.1021/cg400735f

![Synthesis and structure of µ2-oxo-bis [(pentafluoropropionato)-tris(5-bromo-2-methoxyphenyl)antimony] Synthesis and structure of µ2-oxo-bis [(pentafluoropropionato)-tris(5-bromo-2-methoxyphenyl)antimony]](/file/cover/147233139/synthesis-and-structure-of-2-oxo-bis-pentafluoropropionatotris.png)
