Synthesis and structure of 6-amino-5-nitro-2-propargylsulfanyl-4(3H)-pyrimidinone
Автор: Artemeva E.V., Petrova K.Yu.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Органическая химия
Статья в выпуске: 1 т.14, 2022 года.
Бесплатный доступ
6-Amino-5-nitro-2-propargylsulfanyl-4(3 H )-pyrimidinone (1) has been synthesized for the first time by the reaction of 6-amino-5-nitroso-2-propargylsulfanyl-4(3 H )-pyrimidinone with tert -butyl hydroperoxide at equimolar ratio of the reactants leading to selective oxidation of nitroso group to a nitro one. The product is a crystalline substance, soluble in aromatic hydrocarbons, resistant to air oxygen and moisture. The compound has been characterized by IR and 1H NMR spectroscopy and X-ray diffraction analysis. The IR spectrum of 1 contains absorption bands at 3290 (NH2, st ), 2855 (S-CH2, st ), 1684 (C=O, st ) and 1595 (NO2, st ) cm-1. In the 1H NMR spectrum of 1 there are singlets of SCH2 protons at 3.87 ppm and С≡CH at 3.12 ppm. Downfield, there are singlets of NH2 group protons at 7.77 ppm and pyrimidine ring NH protons at 10.89 ppm. According to X-ray diffraction analysis data, in the crystal there are two types of crystallographically independent molecules, geometrical parameters of which are slightly different. X-ray diffraction data show that the crystal cell contains molecules of the used solvent, dimethylformamide. The planes of the nitro and thio groups are parallel to the plane of the heterocyclic fragment. C-NO2 and C-NH2 bond lengths are 1.452(2) and 1.317(2) Å, which is usual for compounds of these classes. The CAr-S distance is 1.748(2) Å. The structural arrangement of molecules in a crystal is due to O···H hydrogen bonding (1.91(2)-2.70(1) Å) and intermolecular N···O interactions (2.767(3)-2.984(3) Å). Also, there are face-to-face stacking interactions, the distances between centroids of aromatic rings are 4.13-4.46 Å.
6-amino-5-nitro-2-propargylsulfanyl-4(3h)-pyrimidinone, oxidation, structure, x-ray diffraction analysis, ir spectroscopy, 1h nmr spectroscopy
Короткий адрес: https://sciup.org/147236628
IDR: 147236628 | УДК: 547.854.83+547.859.3 | DOI: 10.14529/chem220110
Текст научной статьи Synthesis and structure of 6-amino-5-nitro-2-propargylsulfanyl-4(3H)-pyrimidinone
Pyrimidine derivatives are known to be biologically active, with antiviral [1, 2], antitumor [3–5], antimycotic [6–8], HIV-1 [9–11] and anti-COVID-19 [12] activities.
Nitrosobenzenes can be oxidized to nitro compounds by oxidizing agents such as permanganate, chromate, hexacyanoferrate, Caro's acid [13], nitric acid, nitrogen dioxide and nitrogen monoxide [14], hypochlorite and hydrogen peroxide in alkaline medium [15], and in a mixture of hydrogen peroxide with nitric acid in glacial acetic acid as a solvent [16]. Nitrosobenzene can be oxidised by peroxocarboxylic acids. Thus, peroxotrifluoroacetic acid reacts in a solution of methylene chloride when boiling with nitrosobenzene to form nitrobenzene [17]. 5-Nitrosopyrimidine is oxidized to 5-nitropyrimidine by hydrogen peroxide using trifluoroacetic acid as a solvent [18].
In the present paper, we continue the discussion of substituted nitrosobenzene oxidation reactions on the example of 6-amino-5-nitroso-2-propargylsulfanyl-4( 3H )-pyrimidinone interaction with tert butyl hydroperoxide.
Experimental
The starting 6-amino-5-nitroso-2-propargylsulfanyl-4( 3H )-pyrimidinone was previously obtained by 6-amino-2-propargylsulfanyl-4( 3H )-pyrimidinone nitrosation according to the known procedure [19].
Synthesis of 6-amino-5-nitro-2-propargylsulfanyl-4( 3H )-pyrimidinone (1).
6-Amino-5-nitroso-2-propargylsulfanyl-4( 3H )-pyrimidinone (0.04 g, 0.19 mmol) was dissolved in 10 ml of dimethylformamide, then 70 % aqueous solution of tert -butyl hydroperoxide (0.017 g,
0.19 mmol) was added. The mixture was kept for 24 h at 20 °C. After the solvent evaporation, the oily dark green product was recrystallized from benzene. As a result, 0.023 g (40 %) of beige crystals of 1 with MP > 300 °C, with decomposition, was obtained.
IR spectrum, ν, сm–1: 3290 (NH 2 ), 2923, 2855 (S–H), 1684 (C=O), 1653, 1595 (NO 2 ), 1517, 1507, 1419, 1457, 1448, 1437, 1395, 1388, 1363, 1283, 1247, 1223, 1180, 1122, 1066, 1015, 976, 804, 486, 444, 419.
-
1H NMR (500 MHz, DMSO- d 6 ) δ 10.89 (s, 1H, NH), 7.77 (s, 2H, NH 2 ), 3.87 (s, 2H, SCH 2 ), 3.12 (s, 1H, С≡CH).
Found, %: С 40.10, Н 4.25. For C 20 H 26 N 10 O 8 S 2 calculated, %: С 40.13, Н 4.38.
IR spectrum of compound 1 was recorded on a Shimadzu IRAffinity-1S FTIR-spectrometer; sample was prepared by pelletting with KBr (absorption region 4000–400 cm–1).
-
1H NMR spectrum was recorded on a Bruker DRX-500 spectrometer (500 MHz) in DMSO, the internal standard was TMS.
X-ray diffraction analysis of crystalline substance 1 was performed on a Bruker D8 QUEST automatic four-circle diffractometer (Mo K α -emission, λ 0.71073 Å, graphite monochromator).
Data collection and editing, unit-cell parameters refinement, and correction for absorption were carried out in SMART and SAINT-Plus software [20]. All calculations aimed at solving and refining the structure of compound 1 were performed in SHELXL/PC [21] and OLEX2 software [22]. Structure 1 was determined by direct methods and refined with the least squares method in the anisotropic approximation for non-hydrogen atoms.
Crystal Data for C 20 H 26 N 10 O 8 S 2 ( M 598.63 g/mol): triclinic, space group Pī, a 7.267(7) Å, b 12.857(12) Å, c 15.417(15) Å, α 85.89(7)°, β 88.33(4)°, γ 75.16(4)°, V 1389(2) Å3, Z 2, μ Mo 0.254 mm–1, D calc 1.432 g/cm3, 31188 reflections measured, 5646 unique reflections ( R int 0.0410), the number of refinement variables 386, GOOF 1.029 , R factors for F 2 > 2 σ ( F 2): R 1 0.0364, wR 2 0.0894, R factors for all reflections R 1 0.0553, wR 2 0.0991.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 2050940 for compound 1; ; .
Results and Discussion
In the present work, the synthesis of 6-amino-5-nitro-2-propargylsulfanyl-4( 3H )-pyrimidinone has been carried out by the reaction of 6-amino-5-nitroso-2-propargylsulfanyl-4( 3H )-pyrimidinone with tert butyl hydroperoxide at room temperature at an equimolar ratio of reactants:
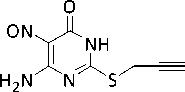
t -BuOOH
O2N
H2N
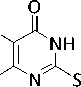
+ t -BuOH
Compound 1 is a crystalline substance, soluble in aromatic hydrocarbons, resistant to moisture and air oxygen.
Structure 1 has been determined by X-ray diffraction analysis and confirmed by IR and NMR 1H spectroscopy. Suitable for X-ray diffraction analysis crystals were obtained after recrystallization of the reaction product from benzene.
In the IR spectrum of compound 1 , there are absorption bands at 3290 cm–1 (NH 2 , st ), 2855 cm–1 (S–CH 2 , st ), 1684 cm–1 (C=O, st ) [23]. The broad absorption band of the N=O group with a maximum at 1559 cm–1, characteristic for the starting compound [19], is absent in 1 . Instead, there is an intensive band at 1595 cm–1 due to NO 2 -group stretching vibrations [23].
The 1H NMR spectrum of 1 contains singlets of the SCH 2 protons at 3.87 ppm and С≡CH at 3.12 ppm. Downfield, there are signals of NH 2 group protons at 7.77 ppm. In an even farther downfield region, there is a singlet of pyrimidine ring NH protons at 10.89 ppm.
According to X-ray diffraction data, in crystal 1 there are two types of crystallographically independent molecules a and b , the geometric parameters of which are equal within the error limits, therefore, in the following, we discuss the structural data of molecule 1 a . The substance crystallizes in the form of a solvate with dimethylformamide (Fig. 1).
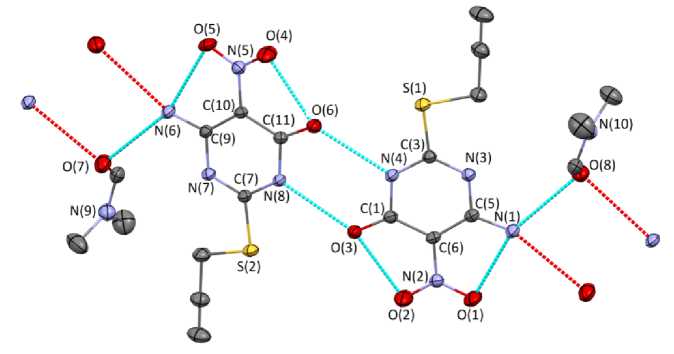
Fig. 1. Structure 1 ( a and b ) showing thermal ellipsoids at 30% probability. Hydrogen atoms have been omitted for clarity. Selected bond lengths (Å) and angles (°): C(5)–N(1) 1.317(2), C(6)–N(2) 1.452(2), C(3)–S(1) 1.748(2), C(3)–N(4) 1.352(2), C(3)–N(3) 1.303(3), N(2)–O(1) 1.223(2), N(2)–O(2) 1.209(2); S(1)C(3)N(3) 120.4(1), C(3)N(3)C(8) 117.7(1), O(1)N(2)O(2) 122.3(2), C(2)S(1)C(3) 100.3(1)
The compound has a struc ture simila r to the struc tur e of 2 -amino-5-nitropyrimidine and 6-methylamino-4-methylthio-5-nitro-2- p he n y lp y r i mi dine [24, 25]. The nitro- and thio- group planes are coplanar with the pla ne of the he te r oc y c lic f r a gm e nt. T he le n gths of the e xocy c lic C –NO 2 and C–NH 2 bonds are 1.452(2) and 1. 317( 2) Å, whic h is usu a l f or c o mpounds of the se c la ss e s. T he C Ar –S distance is 1.748(2) Å.
T he s tr u c tu r al or g a niza t ion of molecules in a crystal is due to hydrogen bonds O· · · H ( 1. 91 ( 2) – 2 . 70( 1 ) Å) ( Fig . 2) a n d int e rmolecular interactions between nitrogen and oxy g e n a toms N· · · O (2.767(3)– 2. 984(3) Å) ( Fig . 3) . T h e dis ta nce s betwe e n nitr og e n a nd oxygen atoms are slightly less than t he sum of Va n d e r W a als r adi i of N a nd O a toms (3. 15 Å) [26]. There are also face-to-face stacking i nt e r ac t ions, th e di stanc es b e twe e n c e n tr o ids of a r oma ti c r i ng s a r e 4. 13 –4.46 Å.
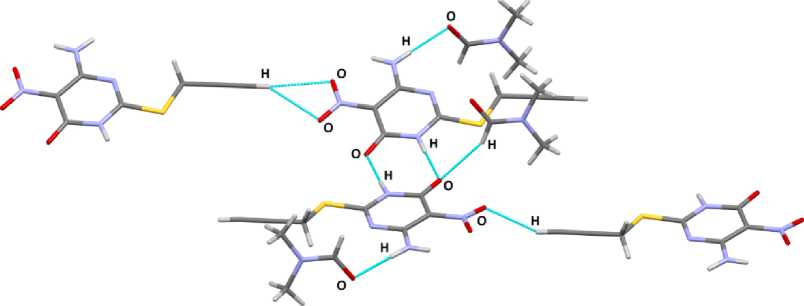
Fig. 2. Hydrogen bonds O···H in 1. Selected bond lengths (Å): 1.91(2), 1.97(2), 2.55(2), 2.59(3), 2.60(2), 2.66(2), 2.70(1)
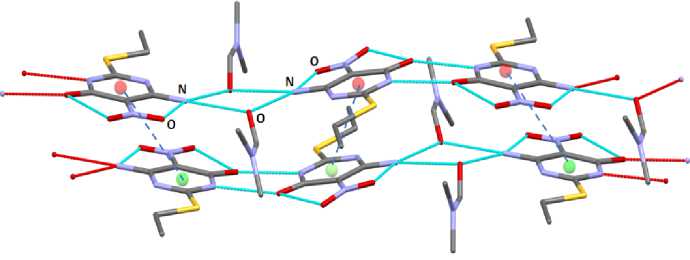
Fig. 3. The intermolecular interactions in 1. Selected bond lengths (Å): N···O (2.767(3), 2,788(3), 2.924(3), 2.984(3))
Conclusion
The oxidation reaction of 6-amino-5-nitroso-2-propargylsulfanyl-4( 3H )-pyrimidinone with tert butyl hydroperoxide at equimolar ratio in dimethylformamide leads to formation of 6-amino-5-nitro-2-propargylsulfanyl-4( 3H )-pyrimidinone, the structural organization of which is due to hydrogen bonds and other short contacts as well as stacking interactions.
Acknowledgments
We are grateful to V.V. Sharutin for the X-ray diffraction analysis.
Список литературы Synthesis and structure of 6-amino-5-nitro-2-propargylsulfanyl-4(3H)-pyrimidinone
- Togninelli A., Carmi C., Petricci E., Mugnaini C., Massa S., Corelli F., Botta M. Solution-phase Parallel Synthesis of S-DABO Analogues. Tetrahedron Lett., 2006, vol. 47, no. 1, pp. 65–67. DOI: 10.1016/J.TETLET.2005.10.142.
- Janeba Z., Holý A., Snoeck R., Andrei G., Clercq E. De, Balzarini J. Synthesis and Antiviral Evaluation of 3-(2,3-Dihydroxypropyl)furo[2,3-d]pyrimidin-2(3H)-ones. Antiviral Res., 2010, vol. 1, no. 86, p. A57. DOI: 10.1016/J.ANTIVIRAL.2010.02.442.
- Ondi L., Lefebvre O., Schlosser M. Brominated 4-(Trifluoromethyl)pyrimidines: A Convenient Access to Versatile Intermediates. European J. Org. Chem., 2004, vol. 2004, no. 17, pp. 3714–3718. DOI: 10.1002/EJOC.200400209.
- Novakov I.A., Orlinson B.S., Brunilin R. V., Nawrozkij M.B., Savel’ev E.N., Novikova G.A. Synthesis of Novel N2-Adamantyl Derivatives of 2-Amino-6-methyl-4(3H)-pyrimidinone as PotentialActivators of Tumor Necrosis Factor (TNF) Release. Chem. Heterocycl. Compd., 2006, vol. 42, no. 10, pp. 1331–1333. DOI: 10.1007/S10593-006-0243-7.
- Ali Abu-Hashem A., Abdel Raouf Hussein H. Synthesis and Antitumor Activity of New Pyrimidine and Caffeine Derivatives. Lett. Drug Des. Discov., 2015, vol. 12, no. 6, pp. 471–478. DOI: 10.2174/1570180812666150429234237.
- Fathalla O.A., Awad S.M., Mohamed M.S. Synthesis of New 2-Thiouracil-5-sulphonamide Derivatives with Antibacterial and Antifungal Activity. Arch. Pharmacal Res., 2005, vol. 28, no. 11, pp. 1205–1212. DOI: 10.1007/BF02978199.
- Wang S.-C., Wan F.-X., Liu S., Zhang S., Jiang L. Synthesis and Antifungal Activity Evaluation of Novel Substituted Pyrimidine-5-carboxamides Bearing the Pyridine Moiety. J. Chinese Chem. Soc., 2018, vol. 65, no. 4, pp. 445–451. DOI: 10.1002/JCCS.201700310.
- Miyamoto Y. Synthesis and Antifungal Activity of [l,2,4]Triazolo-[1,5-c]-pyrimidine Derivatives. J. Pestic. Sci., 1986, vol. 11, no. 1, pp. 39–48. DOI: 10.1584/jpestics.11.39.
- Sim O.G. Sintez Biologicheski Aktivnyh Novyh 5-Zameshchennyh Proizvodnyh 2-Aminopirimidin-4(3H)-ona [Synthesis of Biologically Active New 5-Substituted Derivatives of 2-Aminopyrimidine-4(3H)-one]. Avtoref. dis. … kand. farm. nauk. Volgograd, 2006. (in Russ)
- Mohamed A.M., El-Sayed W.A., Ibrahim A.A., Abdel-Hafez N.A., Ali K.A.K., Mohamed S.F. Recent Trends in the Chemistry of [1,2,4]Triazole[1,5-a]pyrimidines. Org. Prep. and Proc. Int., 2021, vol. 53, no. 3, pp. 211–239. DOI: 10.1080/00304948.2020.1871310.
- Kang D., Ruiz F.X., Sun Y., Feng D., Jing L., Wang Z., Zhang T., Gao S., Sun L., Clercq E. De, Pannecouque C., Arnold E., Zhan P., Liu X. 2,4,5-Trisubstituted Pyrimidines as Potent HIV-1 NNRTIs: Rational Design, Synthesis, Activity Evaluation, and Crystallographic Studies. J. Med. Chem., 2021, vol. 64, no. 7, pp. 4239–4256. DOI: 10.1021/ACS.JMEDCHEM.1C00268.
- Abu-Zaied M.A., Elgemeie G.H., Mahmoud N.M. Anti-Covid-19 Drug Analogues: Synthesis of Novel Pyrimidine Thioglycosides as Antiviral Agents Against SARS-COV-2 and Avian Influenza H5N1 Viruses. ACS Omega, 2021, vol. 6, no. 26, pp. 16890–16904. DOI: 10.1021/ACSOMEGA.1C01501.
- Kuhn R., van Klaveren W. Darstellung von o-Dinitro-Verbindungen. Berichte Der Dtsch. Chem. Gesellschaft (A B Ser.), 1938, vol. 71, no. 4, pp. 779–780. DOI: 10.1002/cber.19380710414.
- Park J., Choi Y.M., Dyakov I. V., Lin M.C. An Experimental and Computational Study of the Thermal Oxidation of C6H5NO by NO2. J. Phys. Chem. A, 2002, vol. 106, no. 12, pp. 2903–2907. DOI: 10.1021/jp0135353.
- Bamberger E. Ueber Die Oxydation Wässriger Arylhydroxylaminlösungen Durch Den Luftsauerstoff. Berichte Der Dtsch. Chem. Gesellschaft, 1900, vol. 33, no. 1, pp. 113–122. DOI: 10.1002/cber.19000330113.
- Zuman P., Shah B. Addition, Reduction, and Oxidation Reactions of Nitrosobenzene. Chem. Rev., 1994, vol. 94, no. 6, pp. 1621–1641. DOI: 10.1021/cr00030a007.
- Boyer J.H., Ellzey E. Oxidation of Nitrosoaromatic Compounds with Peroxytrifluoroacetic Acid. J. Org. Chem., 1959, vol. 24, no. 12, p. 2038. DOI: 10.1021/jo01094a616.
- Taylor E.C., McKillop A. A New Synthesis of 5-Nitropyrimidines. J. Org. Chem., 1965, vol. 30, no. 9, pp. 3153–3155. DOI: 10.1021/jo01020a067.
- Kim D.G., Osheko K.Y., Frolova T.V. Halocyclization of 2-Allyl(propargyl)sulfanyl-6-aminopyrimidin-4(3H)-ones. Russ. J. Org. Chem. 2017, 2018, vol. 53, no. 12, pp. 1899–1902. DOI: 10.1134/S1070428017120235.
- Bruker (2000) SMART. Bruker Molecular Analysis Research Tool, Versions 5.625 Bruker AXS, Madison, Wisconsin, USA.
- Bruker (2000) SAINTPlus Data Reduction and Correction Program, Versions 6.02a, Bruker AXS, Madison, Wisconsin, USA.
- Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A.K., Puschmann H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr., 2009, vol. 42, no. 2, pp. 339–341. DOI: 10.1107/S0021889808042726.
- Pretsch E., Bühlmann P., Badertscher M. Structure Determination of Organic Compounds: Tables of Spectral Data Structure Determination of Organic Compounds: Tables of Spectral Data. Springer Science & Business Media, Berlin, Heidelberg, 2009, p. 433. DOI: 10.1007/978-3-540-93810-1.
- Pomés Hernández R., Duque Rodríguez J., García Trimiño M.I., Novoa de Armas H., Toscano R.A. 6-Methylamino-4-methylthio-5-nitro-2-phenylpyrimidine. Acta Crystallogr. Sect. C Cryst. Struct. Commun., 1995, vol. 51, no. 7, pp. 1392–1394. DOI: 10.1107/s0108270194013831.
- Aakeröy C.B., Nieuwenhuyzen M., Price S.L. Three Polymorphs of 2-Amino-5-nitropyrimidine: Experimental Structures and Theoretical Predictions. J. Am. Chem. Soc., 1998, vol. 120, no. 35, pp. 8986–8993. DOI: 10.1021/ja981122i.
- Batsanov S.S. Van Der Waals Radii of Elements. Inorg. Mater., 2001, vol. 37, no. 9, pp. 871–885. DOI: 10.1023/A:1011625728803.


