Synthesis of self-healing microcapsules based on dicyclopentadiene
Автор: Cherkashina N.I., Pavlenko V.I., Ruchiy A.Yu., Serebryakov S.V., Barinov R.A.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Application of nanomaterials and nanotechnologies in construction
Статья в выпуске: 2 Vol.17, 2025 года.
Бесплатный доступ
Introduction. The study aims to explore the possibility of creating microcapsules using a carbamide-formaldehyde shell (coat) that contains dicyclopentadiene. Materials and methods. The following reagents were used to synthesize carbamide-formaldehyde microcapsules: distilled water; carbamide (urea) with a purity of 99%; technical formalin; sodium hydroxide (Pro Analysis «PA»); neem seed oil (NSO); polysorbate Twin-80; dicyclopentadiene with a purity of 98%; absolute ethyl alcohol (dehydrated); hydrochloric acid (Pro Analysis «PA»). Results and discussion. Organic microcapsules were obtained by polymerization of carbamide-formaldehyde resin. Filling of microcapsules with dicyclopentadiene has been carried out by establishing equilibrium between concentrated solution of dicyclopentadiene in alcohol and capsule contents. Average size of capsules was ~100 μm. Capsules have spherical shape and solid shell. Fourier-transform IR (FTIR) spectrum confirmed required compositions of capsule shell and filler. Analysis of DSC curve (Differential scanning calorimetry curve) showed that filling of capsules with dicyclopentadiene was successful. Conclusion. The result of the research done is the successful synthesis of microcapsules using a carbamide-formaldehyde shell. This synthesis of microcapsules and its application in building materials technology opens up new prospects for creating composites with selfhealing properties.
Self-healing, self-restoration, formaldehyde, carbamide (urea), dicyclopentadiene, microcapsules, synthesis
Короткий адрес: https://sciup.org/142243956
IDR: 142243956 | DOI: 10.15828/2075-8545-2025-17-2-189-200
Текст научной статьи Synthesis of self-healing microcapsules based on dicyclopentadiene
Original article
Черкашина Н.И., Павленко В.И., Ручий А.Ю., Серебряков С.В., Баринов Р.А. Синтез самозалечивающихся микрокапсул на основе дициклопентадиена. Нанотехнологии в строительстве. 2025;17(2):189–200. – EDN: XIVHFT.
Modern research into materials of various compositions with a self-healing or self-restoring effect is a promising area of study in science [1–3]. These compositions are used not only in building materials [4–7], but also in bioengineering [8] and electronics [9–10], ensuring durability and resistance of structures made from them to mechanical damage.
Self-healing is the property of a material or system to partially or completely restore its original structure and functionality after physical and mechanical impacts (damage) [11–14]. Self-healing materials are able to restore their integrity [15–16] after damage due to the introduction of micro- and/or nanocapsules containing healing agents and catalysts into their structure, which react with subsequent hardening when a defect occurs [17–18]. The shell of the microcapsules can be made from both mineral [19] and polymer [20] materials and must have certain strength characteristics [21]. It must be strong enough not to be damaged during the manufacturing process of composite materials and while also being able to break down when various types of defects occur. Analysis of literature data has shown that the choice of components for the synthesis of microcapsules depends on the intended purpose and area of their future use [22, 23].
In the study [24], scientists described a method for creating self-healing porous concrete composites. This self-healing effect is achieved through the high-density structure of the material and the presence of an active mineral, iron sulfide, in the pores of the filler. When a defect forms in the finished product, the pores become damaged. Iron sulfide reacts with hydrated water in the filler to form ferrous ettringite, a hardening substance that closes the crack.
In the article [25], researchers examined the mechanisms of self-healing of polymer membranes used for water filtration. The scientists identified two types of self-healing: external and internal. External healing is dependent on microcapsules with a healing agent and a catalyst built into the membrane matrix, Internal healing is dependent on dynamic bonds between polymers that trigger autonomous healing through reversible chemical interactions (when bonds are broken, conformational changes occur and lead to contact between the ends of low-molecular reactive chains).
In the study [26], scientists investigated the physical properties of a self-healing composite based on epoxy resin with the addition of carbamide-formaldehyde microcapsules with a dicyclopentadiene monomer, multi-walled carbon nanotubes (MWCNTs) and carbon fibers. They have found that this modification method significantly increases the tensile strength of the material without compromising the self-healing efficiency (90%) compared to unmodified samples (72%). The material also has shown promising values of a higher glass transition temperature (85 °C) in combination with improved curing efficiency (90%), which can be considered the novelty of this research.
The study [27] presents a method for creating selfhealing polyurethane composite fibers with the addition of environmentally friendly cellulose nanocrystals (CNC) and investigates some physical properties of this material. Compared to polyurethane fibers without CNC additive, the elongation at break and tensile strength of the composite fibers have increased by 34% and 18%, respectively. After the cutting test, the self-healing poly-
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION urethane composite fibers with CNC showed no cracks on the surface, and their elongation at break and tensile strength increased by 57% and 128% respectively.
The aim of the study in the article [28] is to obtain composite hydrogels (PBLH) based on polyvinyl alcohol (PVA), borate (B), ε-poly-L-lysine (EPL) and hyaluronic acid (HA) with ultra-fast self-healing ability, elasticity and moldability by an efficient one-step method. The fabricated hydrogels exhibited outstanding characteristics of fast self-healing, stretchability and moldability. They could completely recover within one minute and take various shapes as specified.
Thus, the self-healing effect can be achieved in both mineral and organic building materials. This helps to extend the service life of production elements, reduce the costs of their maintenance and repair, and improve the safety of the structure or product as a whole. The aim of this research is to synthesize self-healing microcapsules based on a carbamide-formaldehyde shell with dicyclopentadiene. This article describes the process of producing microcapsules, analyzes their granulometric composition, and investigates the microstructure of the synthesized product. Additionally, the results of differential scanning calorimetry (DSC) curves of microcapsules without filler and with dicyclopentadiene filling has been carried out.
MATERIALS, EQUIPMENT, RESEARCH METHODS
Materials
To obtain carbamide-formaldehyde microcapsules, we used the following reagents in our study: distilled water in accordance with GOST 58144-2018; carbamide (urea) with a purity of 99% produced by Macklin Inc., China; technical formalin in accordance with GOST 1625-89 produced by LenReaktiv JSC, Russia, St. Petersburg; sodium hydroxide “ChDA” produced by Kaustik JSC, Russia, Volgograd; neem oil (NSO – Neem Seed Oil) produced by Shree Baidyanath Ayurved Bhawan Pvt., Ltd., India; Twin-80 polysorbate produced by Jaikisan Agrotech India Pvt., Ltd., India; dicyclopentadiene with a purity of 98% produced by Shanghai Acmec Biochemical Technology Co., Ltd.; absolute ethyl alcohol produced by RFK JSC, Russia, Moscow; hydrochloric acid “ChDA” produced by JSC “Caustic”, Russia, Volgograd.
Equipment and research methods
The mass of the initial components and synthesized products was measured using laboratory scales from BK-600, manufactured by JSC MASSA-K, Russia, with a reading resolution of 0.01 grams.
To carry out the synthesis, we used a magnetic stirrer with heating and precise temperature setting 5Drops-HSS-2 “HS5S full set” manufactured by “Joanlab Equip- ment Co., Ltd.”, China. The maximum heating temperature is 260 °C.
The mixing of the synthesized components was carried out using a laboratory overhead stirrer “EUROSTAR 20 high speed digital” manufactured by “NV-Lab”, Russia. This device has a rotation frequency range from 0 to 150 rpm, as well as 6000 rpm.
To obtain deionized water, we used a DV-1M water deionizer manufactured by TsvetKhrom LLC, Russia, with a specific conductivity at 25 °C of less than 0.1 µS/cm and a capacity of up to 10 l/h.
Granulometric analysis of the obtained microcapsules was carried out on a laser diffraction analyzer Analysette 22 NanoTec plus manufactured by Fritsch, Germany, with a particle size measurement range of 0.01–2000 µm. The microstructure of the obtained products was analyzed using a Jenaval optical microscope with CarlZeiss JenaGF Planachromat 3.2x/0.06 ∞/-A and CarlZeiss JenaGF Planachromat 12.5x/0.25 ∞/-A objectives manufactured by Carl Zeiss Jena, Germany.
Fourier-transform IR (FTIR) spectra absorption spectrum of the obtained microcapsules was measured using a Sintecon IR10 infrared spectrometer in the range of wave oscillations of 5000 – 500 cm–1 with a spectral resolution of ≤ 2 cm–1.
Differential scanning calorimetry was performed using a synchronous thermal analysis device STA 449 F1 Jupiter manufactured by Netzsch, Germany. The temperature range for analysis was 20–350 °C, with a heating rate of 10 degrees per minute. The test environment was similar to air: 21% O2, 79% Ar.
RESULTS AND DISCUSSION
Description of the technology for creating carbamide-formaldehyde capsules
Formalin weighing 11.1 g with a formaldehyde concentration of ~30%, alkalized to a 10% NaOH solution was mixed with 2.0 g urea and diluted to 200 ml with deionized water preheated to 60 °C. The solution was stirred with an overhead stirrer at 2000 rpm for 2 hours until the solution became slightly cloudy. Then 1 g of neem oil mixture with Twin-80 polysorbate (concentration of the latter is 0.1%) was added to the solution. The temperature in the system increased from 60 to 70 °C. After stirring for 1 hour, the pH of the solution was reduced by 20% hydrochloric acid from 11–10 to 3–4, and the color of the solution changed from yellow to white. Stirring was continued until noticeable polymer particles were formed and the volume of the solution decreased significantly.
The process of carbamide dissolving in formaldehyde is endothermic. The reaction conditions do not affect the production of primary reaction products, which are carbideoxymethylene (methylol) compounds. Carbonic acid
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION diamide (Fig. 1) is theoretically capable of adding 4 formaldehyde molecules, forming tetramethylol urea (Fig. 2).
The reaction rate constants ratio for the production of mono-, di- and trimethylolcarbinol is 9:3:1. The content of tetramethylolcarbamide is ultimately within the error limits and therefore was not taken into account. The mass ratio of carbamide to formaldehyde of 1:2 ensures the optimal equilibrium of the system for the formation of the largest amount of trimethylolcarbamide in the solution. It was also found that with an increase in the content of formaldehyde in the solution, approximately 2.8 moles of formaldehyde will still react per 1 mole of carbamide.
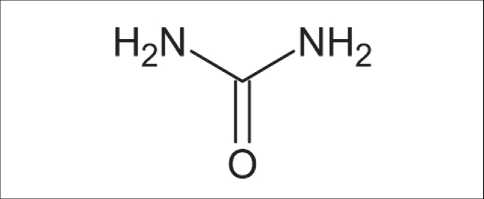
Fig. 1. Structural formula of carbonic acid diamide (urea, carbamide)
Mono- and dimethylolcarbamide have been of the greatest practical importance due to their ability to form polymers of a linear structure. The polymerization reactions of mono- and dimethylolcarbamide are presented below (Fig. 3).
During the curing process of the resin, this reaction also occurred between the free methylol groups. This reaction of polymerization can occur in parallel with the interaction between the ketone and hydroxyl groups (Fig. 3c).
Polymerization of trimethylolcarbamide (Fig. 4) can occur through both hydroxyl and ketone groups. Regardless of which functional groups are involved in the polymerization process, branched polymers are formed during the reaction.
The presence of such branched compounds has had a positive effect on the strength characteristics of the final microcapsules due to the linking of linear structures with each other.
In solution, these reactions occurred when heated in the acidic medium. Typically, carbamide-formaldehyde polymer clusters do not exceed a molecular weight of 700 amu. Due to the presence of methylol groups, the resulting compounds have a high solubility in water. To
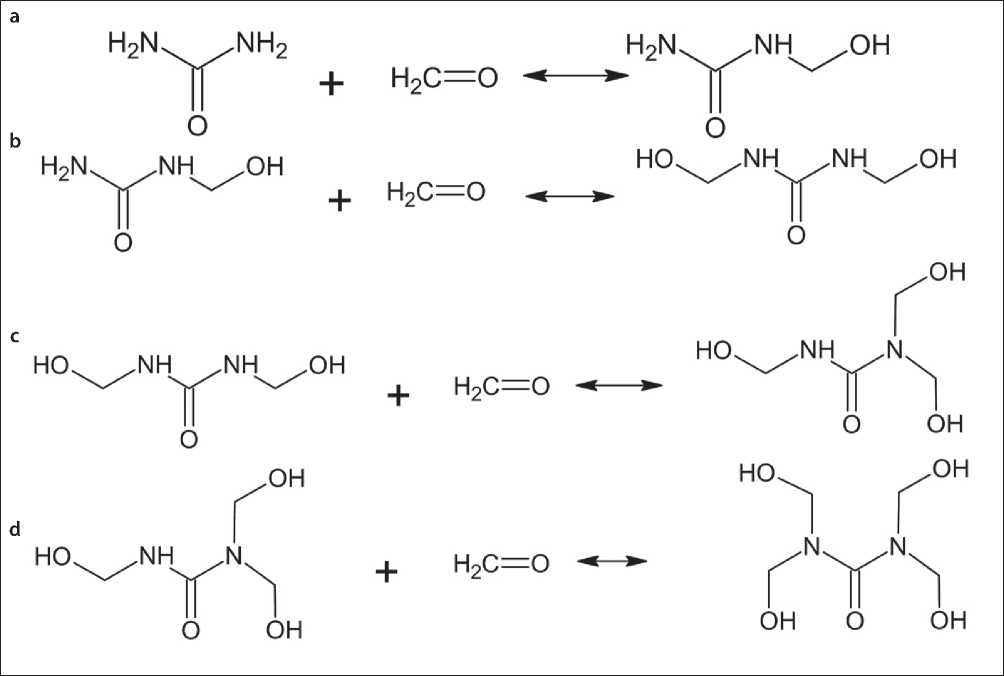
Fig. 2. Reactions for the obtaining of a) monomethylolcarbamide; b) dimethylolcarbamide; c) trimethylolcarbamide; d) tetramethylolcarbamide from carbamide and formaldehyde.
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
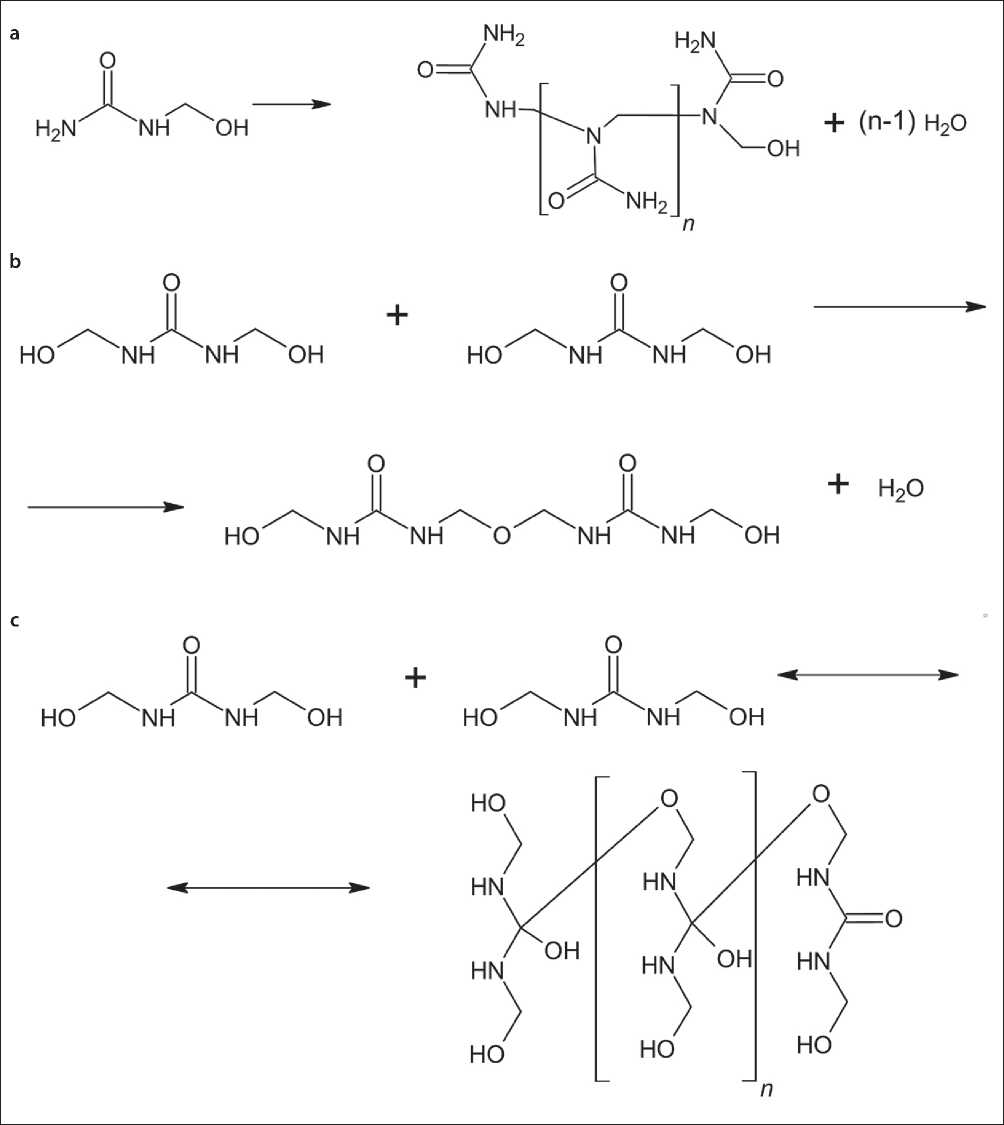
Fig. 3. Polymerization reactions of a) monomethylolcarbamide; b) dimethylolcarbomide; c) dimethylolcarbomide through the interaction of ketone and hydroxyl groups
give them the form of microcapsules, it is necessary to introduce substances with functional groups that can interact with primary amines and form stable polymerization centers. For these purposes, Neem Seed Oil (NSO), which contains nimbin, was introduced into the mixture. Due to its complex spatial structure, it is able to interact with methylolcarbamide through a ketone group, adding a primary amine (Fig. 5).
The size of the microcapsules was controlled by the speed of the overhead mixer and stabilized by Twin-80 polysorbate. The final curing of the carbamide-form-aldehyde resins was carried out in the acidic medium at
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
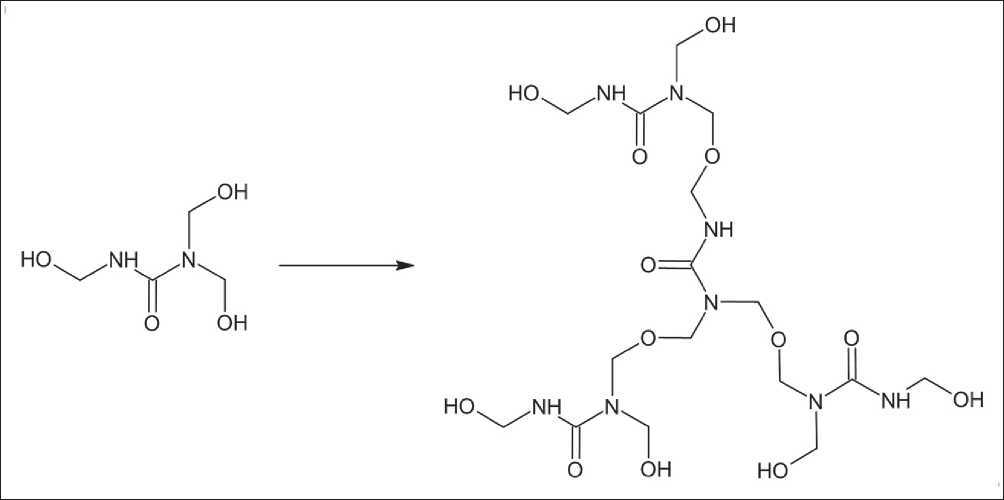
Fig. 4. Polymerization reaction of trimethylolcarbamide
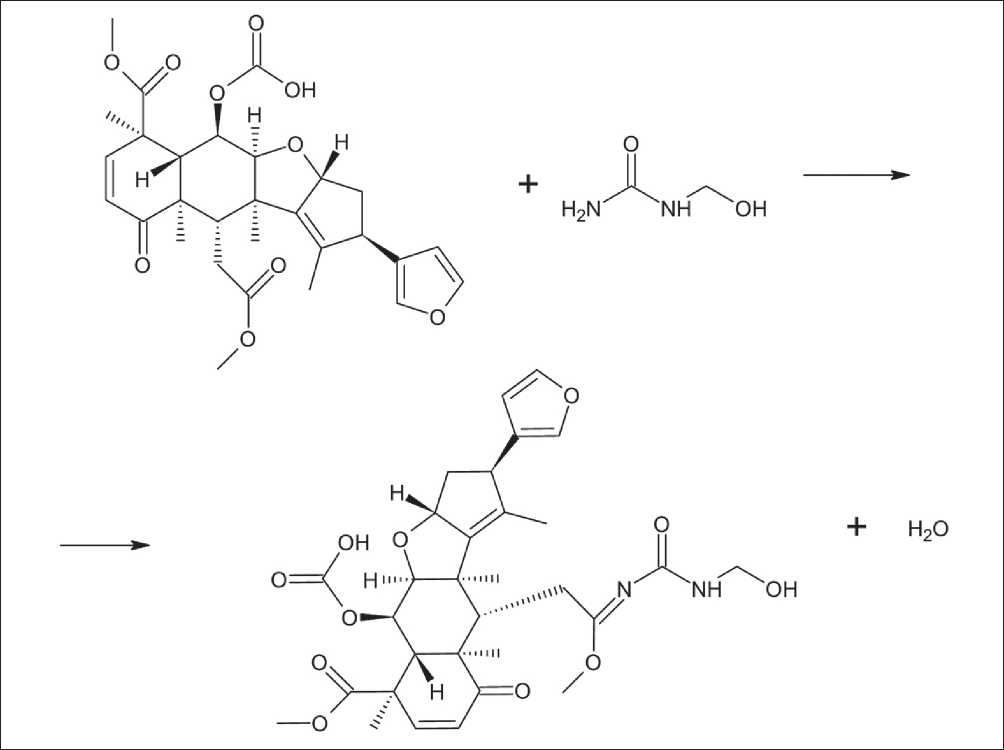
Fig. 5. Reaction of interaction of nimbin with methylolcarbamide
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
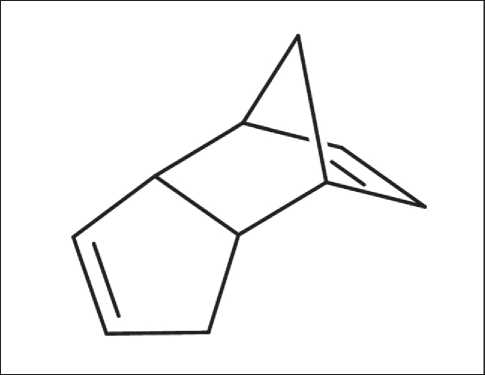
Fig. 6. Structural formula of dicyclopentadiene
pH values of 3–4. The weight of the resulting capsules was 20.4 g.
The next step was nimbin extraction. Extraction was performed in a Soxhlet apparatus with a 64% aqueous solution of ethyl alcohol as a solvent. During the extraction, oil was released from the microcapsules, causing the alcohol to turn white. The capsules were then washed on a polymer sieve (hole size 106 µm) using alcohol and deionized water. A 1.17-fold decrease in the capsule mass was recorded, amounting to 18.6 g. Such an insignificant change in mass is due to the replacement of the capsule contents with a solvent.
The filling of the capsules with functional contents took place in a boiling, saturated (50%) solution of dicyclopentadiene in ethyl alcohol for 6 hours. Dicyclopentadiene has the following structural formula (see Fig. 6).
Granulometric analysis
The result of granulometric analysis of the obtained microcapsules is presented in Fig. 7. Based on the obtained data, the graph shows that the size distribution of the produced carbamide-formaldehyde microcapsules can be described by a trimodal system with the first mode at 0.81 µm, the second mode at 8.80 µm, and the third mode at 105.97 µm. The predominant fraction of microcapsules has the size of ~100 µm. The specific surface area of the microcapsules is 8210 cm2/cm3.
Microscopic analysis
The morphology of the obtained microcapsules was studied by optical microscopy in transmitted light. The result is presented in Fig. 8. No deformation of the microcapsules during the experiments was observed. The spherical particles of the synthesized product with a continuous shell can be clearly seen in Fig 8. It is also worth
9876543210 % ,x laitnereffiD
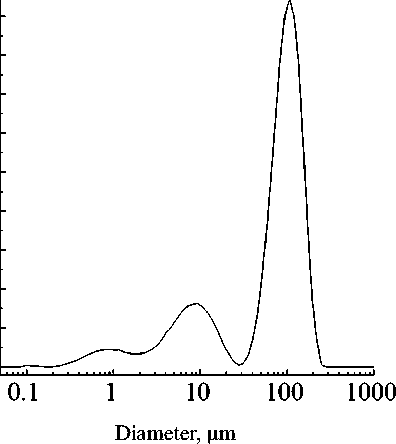
Fig. 7. Granulometric analysis of carbamide-formalde-hyde microcapsules
noting that the average size of the microcapsules is consistent with the granulometric analysis (100 µm).
Fourier Transform Infra-Red spectrum study
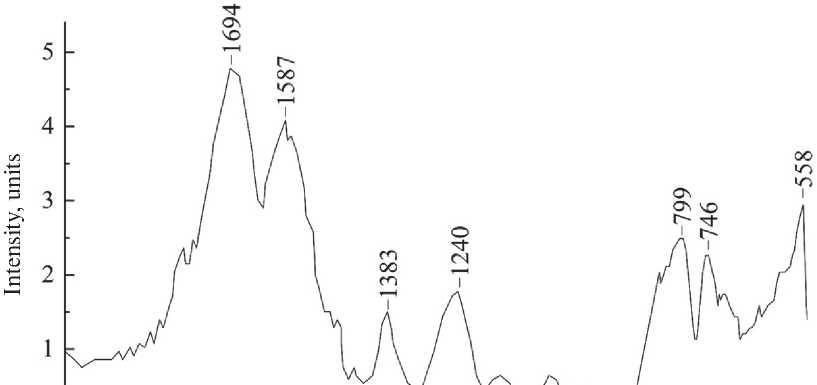
The FTIR spectrum of the carbamide-formaldehyde film (Fig. 9) indicates a high content of amine groups in the polymer structure. A strong peak in the region of 1694 cm–1 indicates NH2 stretching vibrations, which indicates the presence of predominantly monomethylolcarbamide in the polymer system.
The spectrum also shows a peak at 1586 cm–1 (longitudinal deformation of the amide bond), this peak is not typically used to analyze organic substances, but in this case, it indirectly indicates resin polymerization. The height of the peak depends on the concentration and in comparison, with the peak in the region of 746 cm–1 (spiral deformation of the amide bond), which also depends on the concentration, we can judge the presence of carbon frameworks at the ends of the amine bonds that make up the polymer chain. The peaks in the region of 799 and 1240 cm–1 can confidently state the bond of oxygen with carbon, which forms a ketone group.
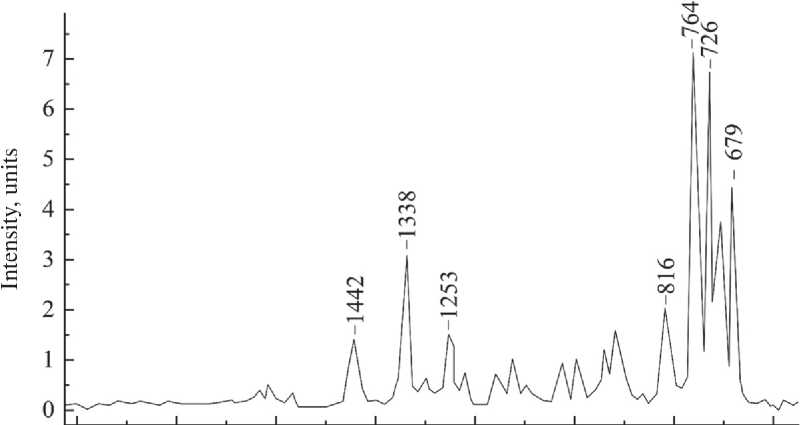
The FTIR spectrum of dicyclopentadiene (Fig. 10) has shown peaks in the region of ~1250-600 cm–1, which are characteristic of cyclic compounds. The presence of peaks at 1338 and 1253 cm–1, provided that the ratio of their intensities is 2:1, indicates the presence of the C–(C)3 bond, which is characteristic of dicyclopentadiene. The high-intensity peak at 764 cm–1 indicates out-of-plane 1,2-substituted bonds in the cycle.
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
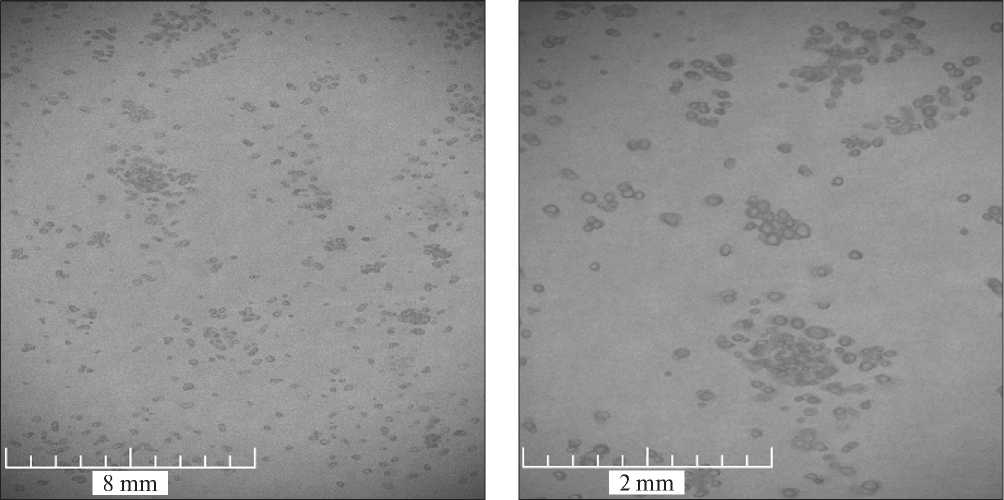
Fig. 8. Microscopic images of carbamide-formaldehyde microcapsules
Q Ll________I________I________I________I________I________I________I________I_______I______±J_ j ________I________I________I_
2000 1800 1600 1400 1200 1000 800 600
Wave number, cm–1
-
Fig. 9. FTIR spectrum of carbamide-formaldehyde film
Differential scanning calorimetry analysis
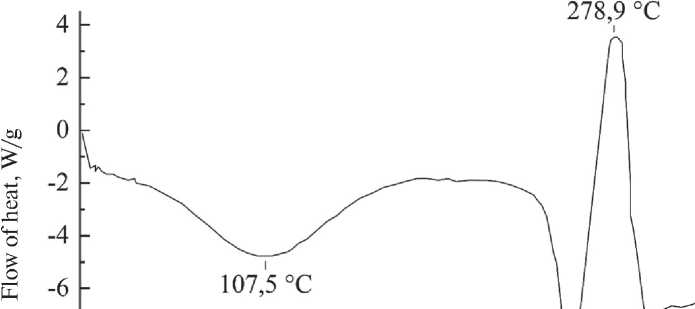
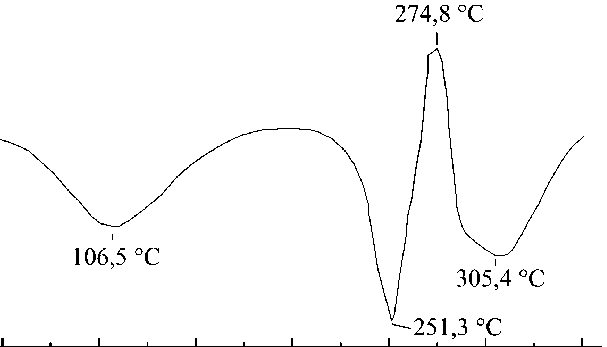
The DSC curve of carbamide-formaldehyde capsules (Fig. 11) shows three endothermic peaks at 107.5 °C; 257.1 °C; 295.9 °C and one exothermic peak at 278.9 °C. The peak at 107.5 °C indicates a phase transition with a slight vitrification of the structure. The subsequent peak at 257.1 °C indicates the complete melting of the peptide with a sharp oxidation at 278.9 °C and degradation with the release of volatile substances at 295.9 °C.
The DSC curve of microcapsules filled with DCPD is shown in Fig. 12 a. The decrease in the heat flow of the endothermic peak at 106.5–107.5 °C is associated with an increase in the evaporation rate of DCPD, which is accompanied by a loss of mass. The decrease in peaks in the range of 251.3–278.9 °C is associated with significant mass
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
2000 1800 1600 1400 1200 1000 800 600
Wave number, cm–1
-
Fig. 10. FTIR spectrum of dicyclopentadiene

-
Fig. 11. DSC curve of carbamide-formaldehyde microcapsules
loss due to the complete combustion of DCPD during the melting of carbamide-formaldehyde resin.
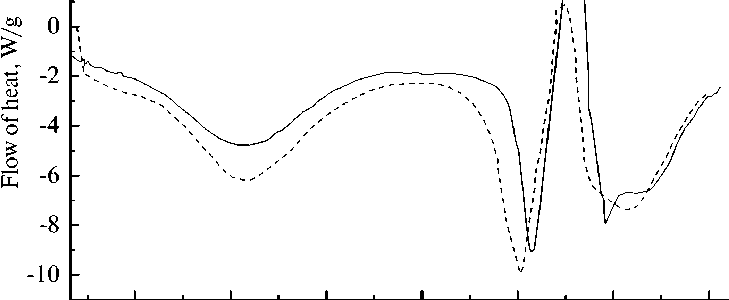
When comparing the two DSC curves (Fig. 12 b), a small difference is visible with the general similarity of the graphs, which indicates successful filling of the capsules with dicyclopentadiene.
CONCLUSION
The result of the experiment performed is the successful synthesis of microcapsules with carbamide-formal-dehyde shell, which is confirmed by FTIR spectroscopy. Microscopic analysis of the obtained product has shown
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
а
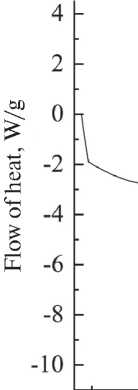
50 100 150 200 250 300 350
Temperature, °С
b
4 - СF film
СF + DCPD
2 -
50 100 150 200 250 300 350
Temperature, °С
Fig. 12. DSC curves: a) carbamide-formaldehyde capsules with dicyclopentadiene; b) comparison of carbamideformaldehyde capsules without filler (СF film) and carbamide-formaldehyde capsules containing dicyclopentadiene (СF + DCPD)
the absence of damage, and that the microcapsules have a solid spherical shell. Analysis of the granulometric composition of the synthesized product has shown that the predominant fraction has a size of ~ 100 µm. Comparative analysis of the curves of differential scanning calorimetry of microcapsules without filler and with DCPD filling showed that the lower value of the heat flow of about 107 °C is associated not only with the phase transition of the microcapsules, but also with an increase in the evaporation rate of DCPD. A decrease in the peak of the heat flow by about 250 °C is due to both the melting of the capsule shell and the incomplete release and evaporation of DCPD. The decrease in the peak around 275 °C and the increase in its half-width can be attributed to the subsequent simultaneous combustion of the heterogeneous system of DCPD and carbamide-formaldehyde resin.
The development of microcapsules with a carbamideformaldehyde shell and their application in building materials opens up new possibilities for creating functional composites with self-healing properties.
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION


