The application of MRNA vectors for CART therapy in vivo
Автор: Kulinich T.M., Bozhenko V.K., Ranjit R., Kaprin A.D.
Журнал: Вестник Российского научного центра рентгенорадиологии Минздрава России @vestnik-rncrr
Рубрика: Обзоры
Статья в выпуске: 4 т.23, 2023 года.
Бесплатный доступ
CAR-T-lymphocyte therapy has revolutionized cancer immunotherapy because genetically modified T cells have made it possible to recognize the necessary tumor antigens. However, genetically modified T cells attack not only cancer cells but also other physiologically functioning cells that express a similar antigen on their cell surface. Another disadvantage of CAR-T-lymphocyte therapy is the associated cost, as mass production of the drug is not possible due to the need to genetically modify the patient's T cells. To address these problems, mRNA can be used to deliver genetic material to T lymphocytes. Since mRNA temporarily expresses its genetic material, side effects can be controlled by adjusting the amount of drug administered. In addition, the manufacturing process does not require the use of the patient's T cells, which means that the drug can be mass produced, reducing its cost.
Car-t, t-lymphocytes, mrna, tumours, immunotherapy
Короткий адрес: https://sciup.org/149145015
IDR: 149145015
Текст научной статьи The application of MRNA vectors for CART therapy in vivo
Different methods of transfection
Prior to mRNA transfection, there were many methods to introduce genetic materials into the T-cells.
They can be classified as viral and non-viral delivery system (Tabl. 1).
Tabl. 1. Classification of transfection methods
|
Viral |
Non-viral |
|
|
Adenovirus-associated viruses |
physical technique |
chemical technique |
|
lentivirus |
electroporation |
calcium phosphate |
|
adenovirus |
needle injection or micro injection |
DEAE Dextran |
|
bacteriophage |
laser irradiation or optical transfection |
lipofection (lipid-mediated or liposome transfection) |
|
gene guns or biolistic transfection |
||
Viral vectors have long been used to deliver genetic material due to their high transfection efficacy, but they have a high probability of suffering from immunogenicity and cellular toxicity [8]. Lentiviral gene transfer is a versatile and powerful method for genetic transduction of many cell lines and primary cells, including "hard-to-transfect" cells. Lentiviral vectors can carry constructs up to 10 kB in size, transduce non-dividing cells, and provide stable transgene expression by integrating into the human genome. However, lentiviral vectors have a low efficiency of gene transfer compared to other viral vectors. Among all viruses, adenovirus-associated viruses have the lowest toxicity and has the advantage of a strong and transient induction of expression of the gene of interest in a wide variety of cell types and organs [9], but it is a time-consuming method and expensive, and its use is limited by its limited capacity to transfer genetic material of about 5.0 kb [10].
The use of bacteriophages is another viral transfection method. In particular, filamentous M13 bacteriophages are being developed as a new type of vector for safe and targeted systemic delivery of transgenes for in vivo applications [11-14]. They have a number of advantages over the use of traditional viral and non-viral vectors; first, their protein coat consists of a repeating protein unit arranged in an alpha-helical array. This gives the phage unlimited DNA packaging capacity, as the capsid coat only needs to stretch to accommodate the transgene. As a result, the bacteriophage is efficient at condensing and packaging DNA. Second, the protein coat has a high tolerance for mutations, allowing easy introduction of peptide ligands to achieve ligand-directed transduction of the desired cell type. In addition, they are safe, as they have long been used in humans to treat bacterial infections and are approved by the Food and Drug Administration (FDA-USA) for use in food preparations. Finally, phage vectors are easy to produce at high titers and low cost, which is highly desirable for large-scale industrial processes [15,16].
Physical methods include electroporation, needle injection, optical transfection, and biolistic transfection. Microinjection is a technique that uses a fine needle to inject nucleic acids directly into the cell nucleus. The advantages of microinjection are precision in dosage and timing of delivery, high transduction efficiency, and low cytotoxicity. However, manual microinjection is labor-intensive and time-consuming, which limits its application to large numbers of cells in a sample [17].
Optical transfection is a technique that uses laser beams to create transient holes in the cell membrane, allowing nucleic acids to enter [18]. It is highly compatible with standard microscopy optics; the most focused point of the laser is aligned with the image plane, allowing the operator to easily observe the cell during transfection. It is a non-contact and aseptic method. The cell culture configuration can therefore remain 'closed' to the external environment during dosing. In contrast, it is challenging to maintain an aseptic environment on a microscopy platform with an "open-top" tissue culture configuration; this configuration is often required for competing techniques that use micromanipulator arms, such as capillary microinjection or single-cell electroporation [19].
The next physical method is biolistic transfection. This is a technique that uses a device that shoots microscopic gold particles coated with nucleic acids into cells [20]. It was originally developed as a method for gene transfer into plants, as it allows transfer across cell walls [21,22], but it is now being recognized as a technique that is much more broadly applicable, and because it can be used for in vivo as well as in vitro transfections, it has tremendous potential for gene therapy [23,24]. It has the advantage of being able to overcome physical barriers (e.g. the stratum corneum of the epidermis), it can be used multiple times on the same sample, it is suitable for co-transfection of two or more DNAs in a single shot, it can be used on large numbers of cells, and it is fast and easy to use [25,26]. The major advantage is that it is highly efficient; a recent study in rat brain cultures showed that this method was 160-fold, 189-fold and 450-fold more efficient than lipofection, electroporation and calcium phosphate precipitation, respectively, in assaying luciferase activity [27]. The main drawback is that the gene gun itself is expensive to purchase, although the cost of consumables is relatively low.
Among the physical techniques, electroporation is the most widely used. In this method, electrical pulses are used to form cell membranes that are transiently permeable for the entry of genes into the cell membrane [28-30]. One of the main advantages of the physical technique is that it has low immunogenicity, since no biological element is used during the procedure [31]. In addition, it is versatile and can be used for all cell types and for transfection of DNA, RNA, mRNA, RNPs or proteins [32]. It is also cost-effective and reduces development costs and time compared to viral-based delivery methods [33].
Chemical transfection techniques include calcium phosphate transfection, DEAE-Dextran transfection, and lipofection.
Phosphate transfection is a method in which the DNA construct is mixed with calcium chloride in a buffered saline/phosphate solution. Since nucleic acids such as DNA and RNA are also negatively charged, they repel each other, inhibiting their uptake by the cell. One way to overcome this challenge is to use positively charged carrier molecules to deliver negatively charged substrates close enough to the cell membrane to be internalized via endocytosis. Calcium phosphate transfection is one of the chemical transfection methods using this principle. When the DNA construct is mixed with calcium chloride in a buffered saline/phosphate solution and incubated at room temperature, DNA-calcium phosphate coprecipitates are formed. Because these coprecipitates can adhere to the plasma membrane, they are thought to facilitate endocytosis. Unfortunately, this method can be toxic to cells (especially primary cells) and transfection efficiency is relatively low compared to most other methods. In addition, the reaction used to generate the coprecipitates is sensitive to slight changes in pH, temperature, and buffer salt concentrations, leading to unreliable results [34].
Another method of chemical transfection is DEAE-Dextran transfection. DEAE-dextran is a polycationic derivative of dextran (a carbohydrate polymer). When mixed with DNA, the resulting complex is brought into contact with the negatively charged plasma membrane by the polymer's excess positive charge. As with calcium phosphate, this proximity to the membrane is thought to facilitate endocytosis into the cell. The major advantages of this technique are its relative simplicity and speed, limited cost, and remarkably reproducible inter- and intraexperimental transfection efficiencies. Disadvantages include inhibition of cell growth and induction of heterogeneous morphological changes in cells. In addition, the serum concentration in the culture medium must be temporarily reduced during transfection [35].
Lipofection is the most commonly used chemical transfection method. The approach involves combining cationic lipids with other molecules to form unilamellar liposomal vesicles that carry a positive charge. The exact molecular mixture has varied over time as lipofection methods have improved. Regardless of the composition of the vesicles, lipofection is designed to package negatively charged molecules such as nucleic acids in a positively charged vesicle so that they can get closer to the cell membrane (where they are presumably taken up by endocytosis). Therefore, you need to mix your construct or molecule of interest with the vesicle mixtures before transfecting your cells. This approach can be successfully used to transfect a wide range of cell types at relatively low cost (although cost and reaction conditions may increase if other polymers and antibody conjugates are added). In addition, lipofection can transfect cells with DNA of any size to achieve both stable and transient transfection, as well as deliver RNA and proteins into cells. The main drawback of the technique is the low transfection efficiency achieved when used for primary cell transfection, stem cell transfection, and when working with suspension cell lines (mainly due to the fact that it can be cytotoxic and relies on cell division for success) [36].
Among all transfection methods, studies have shown that transfection of immune cells in particular is more challenging than transfection of most other primary mammalian cells [37]. The low transfection efficiency in monocytes/macrophages can be attributed to the following reasons. First, there is a very limited chance for pDNA to freely enter the nucleus due to nuclear envelope breakdown during mitosis, as macrophages do not or hardly proliferate [38,39]. Second, these immune cells are equipped with pattern recognition receptors that can recognize nucleic acids as potential foreign and dangerous viral invaders and initiate the inflammatory signaling cascade leading to pDNA degeneration or macrophage apoptosis [40]. Therefore, finding a robust transfection approach to address these issues is highly desirable.
Transfection of mRNA is a promising alternative to pDNA or viral vectors for achieving target protein expression, especially in non-proliferative cells such as primary human cells [41,42]. An advantage of mRNA transfection is that there is no need for the mRNA to enter the nucleus, nor is there a possibility of integration into the host genomic DNA [43]. Thus, this method can be a suitable alternative for transfection of non-proliferative cells [44], including cells of the immune system. In addition, it avoids genotoxicity issues associated with chromosomal insertion of DNA vectors in clinical gene transfer applications. Unlike most pDNA transfection and viral transduction protocols, mRNA transfection results in transient, unstable gene expression. However, transient expression is advantageous for several hit-and-run applications, including current differentiation protocols [45,46].
Structure of car-t cells
These are the recombinants of different parts of the several receptors. Its major components are CD3 ζ of T-cell receptor (TCR) and single-chain variable fragment (scFv) of antibody variable heavy (V H ) and variable light (V L ) chains, fused by a peptide linker [47] (Fig. 1).
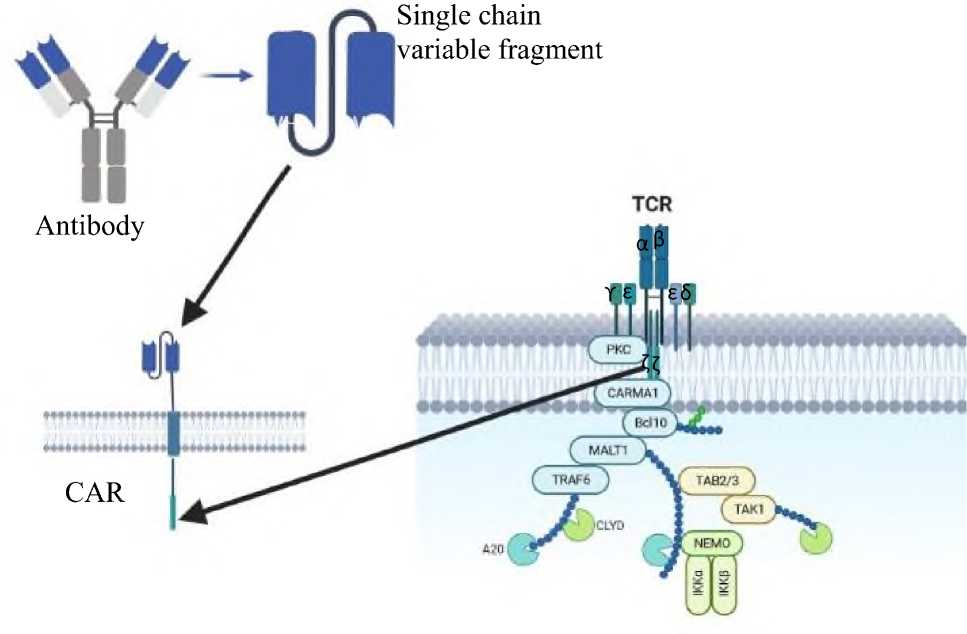
Fig. 1 . Structure of CAR-T cells.
As shown in Fig. 1, the extracellular antigen-identifying domain consists of fragments of monoclonal antibodies (variable zones of light and heavy chains linked to a single chain) that are programmed to identify a specific protein on the cell membrane of cancer cells (e.g., CA-125 for ovarian cancer [48]). This part of the CAR is responsible for recognizing and binding specific tumor-related antigens independent of major histocompatibility complex (MHC) molecules [49,50]. Next, there is a linker protein that crosses the cell membrane and connects the extracellular domain to the intracellular domain. The linker protein is usually derived from CD8, CD3-ζ, CD4, OX40, and H2-Kb [51]. Finally, there is an intracellular domain that consists of a signal transduction component of a T cell receptor (TCR) that directs the signal to the TCR and triggers CAR T cell activation and function [52,53].
Depending on the construction of the intracellular domain, CARs can be classified into 4 different generations. The first generation of CARs was developed in Israel in 1989 and contained only the TCR CD3 ζ chain as an intracellular domain [54]. The main drawback of this generation was that it didn't often respond to antigen stimulation. Therefore, the main difference between the first-generation CARs and others is that the first-generation CARs could not promote CAR T-cell expansion in vivo after reinfusion. On the other hand, subsequent generations contain additional co-stimulatory intracellular domains besides the TCR CD3 ζ chain, which allowed them to enhance the potential of CAR T cells to grow, expand, and ultimately persist in the patient's body [55-57]. To solve this problem, researchers developed the second generation of CARs, where they added an additional co-stimulatory molecule, usually CD28/4-1BB (CD137)/OX40, into the endo-domain with the pre-existing TCR CD3 ζ-chain.The new modification increased the CAR T response to antigen, but inadvertently also increased cell apoptosis [58]. To prevent unanticipated apoptosis of the CAR-T after stimulation, the third generation of CARs was developed that contained a molecule such as CD137 (41BB) as a "survival" factor (Fig. 2). This allowed the CARs to proliferate up to 1000-fold and prolonged their survival up to 3 years in vivo [59]. The latest 4th generation of CARs are called TRUCKs (T cells redirected for universal cytokine killing). In this generation, cytokine genes are transferred into the CAR T design. In this way, CAR-redirected T cells are used as vehicles to produce and release a transgenic product that accumulates in the targeted tissue [60,61].
First Second Third generation generation generation
Fourth generation
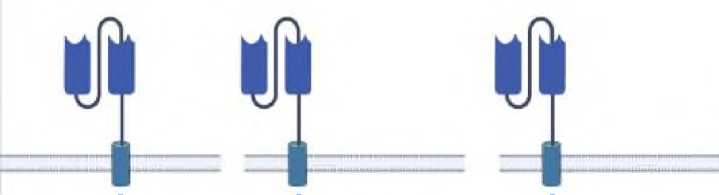
Cell membrane
IL12




Fig. 2. Structures of different generations of CARs.
One of the major drawbacks of CAR-T therapy is its toxicity, especially cytokine release syndrome (CRS) and neurological toxicity [62]. CRS is manifested by an inflammatory response with an unprecedented increase in cytokine levels [63] associated with T-cell activation and proliferation. Symptoms include fever, myalgias, vascular leakage, hypotension, respiratory/renal insufficiency, cytopenias, and coagulopathy [64]. Neurotoxicity is another serious side effect, but the mechanisms involved are poorly understood [65]. CAR T-cell related encephalopathy syndrome (CRES) is a common neurotoxic manifestation that includes a range of symptoms from mild confusion to fatal cerebral edema [66]. However, these side effects are negligible compared to conventional chemotherapy, while the clinical effects are dramatic.
The next problem with CAR T-cell therapy is its cost, which is about half a million dollars [67]. The high cost is associated with the tedious process of introducing genetic material into the T cells and purifying the final product.
Another concern with CAR-T is its on-target, off-target effect. This is related to the fact that tumor-associated antigens (TAAs) are present not only on tumor cells but also on other normal cells. For this reason, other normally functioning cells that express these TAA are also targeted by CAR-T lymphocytes [68].
Benefits of mRNA car-t therapy
The transient effect of mRNA transfection may seem unattractive at first glance, but the technique is capable of solving several serious problems associated with CAR-T therapy. First, in vivo modification of T cells using mRNA technology bypasses the tedious task of PBMC extraction, chemotherapy, and reinfusion of genetically modified T cells. Similarly, the structure of the mRNA is highly customizable, and thus appropriate mRNA for targeted tumors can be rapidly and cost-effectively designed to maximize transfection and translation [69,70]. Another advantage of the mRNA genome delivery system is that the delivered genome is not integrated into the host cell genome, so the side effects associated with it are also transient [71]. Nevertheless, it has been shown that mRNA transfection has the potential to cause fewer on-target/off-target side effects with less toxicity [72-76]. In addition, the level of CAR-T expression and its toxicity is directly proportional to the amount of mRNA delivered to the T cells, so that toxicity such as cytokine release syndrome can be tuned simply by regulating the amount of mRNA injected [72,73].
In a recent study by Rurik et al [77], researchers took the mRNA delivery technology seen in current COVID-19 vaccines and applied it to the basic design of CARs to treat cardiac fibrosis. The mRNA did not integrate into the genome of the T cells, but allowed transient transcription of the mRNA and transient expression of the novel receptors for targeting fibroblast activation protein (FAP) (a marker of activated fibroblasts). The mRMA was then packaged into lipid nanoparticles (Fig. 3), and the surfaces of the lipid nanoparticles were decorated with CD5 targeting antibodies so that they could be taken up by T cells.
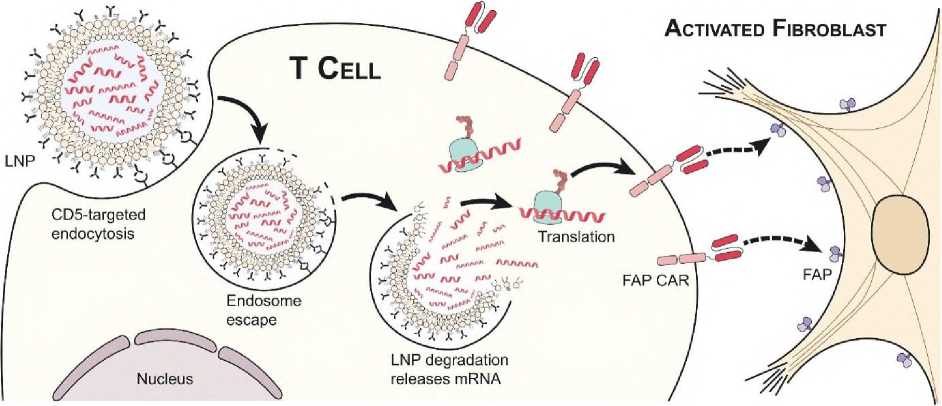
Fig. 3. Schematic diagram of temporary integration of mRNA into the T-cells [77].
Once inside the T cells, the mRNA remained in the T cell cytoplasm but began to express receptors for targeting FAP. The researchers concluded that more than 80% of the T cells expressed the desired CAR after treatment with the tissue culture and could effectively kill target cells with fibroblast activation protein [77].
Short-term CAR-T therapy can be achieved not only by mRNA, but also by other means. When genetic material is delivered by a non-viral method, it can be encoded so that it is not incorporated into the host cell's DNA. In fact, preclinical studies have successfully demonstrated that with electroporation, the introduced genetic material is expressed for only 10 days, after which it disappears, along with all the associated side effects [30].
Conclusion
CAR-T lymphocyte therapy has revolutionized the treatment of cancer because of its ability to specifically target cancer cells. However, their cost and tedious manufacturing process have made them less attractive for use in clinical practice. Similarly, the side effects associated with its use can sometimes be dreadful. To solve both of these problems, the temporary expression of the injected genetic material can kill two rabbits with a single stone-as it would be cost effective and reduce the side effects that occur during the treatment process.
Author Contributions. V.K. Bozhenko: planning and supervision of the project; T.M. Kulinich: сollecting data, analyzing and interpreting results, and preparing the manuscript.; R. Ranjit: analyzing the results and writing the manuscript; A.D. Kaprin: the concept and design of the study. All authors have read and agreed with the version of the manuscript submitted for publication.
Foundation. The publication was supported by the RUDN Strategic Academic Leadership Program. The support had no role in the study, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with patients' rights and bioethics regulations. This review study was based on published papers and therefore did not require ethics committee approval.
Conflict of interest. The authors declare that there is no conflict of interest.
Список литературы The application of MRNA vectors for CART therapy in vivo
- Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018 Oct 1;32(19-20):1267-1284. doi: 10.1101/gad.314617.118.
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020 Nov;20(11):651-668. doi: 10.1038/s41577-020-0306-5.
- Mirzaei HR, Mirzaei H, Lee SY, Hadjati J, Till BG. Prospects for chimeric antigen receptor (CAR) γδ T cells: A potential game changer for adoptive T cell cancer immunotherapy. Cancer Lett. 2016 Oct 1;380(2):413-423. doi: 10.1016/j.canlet.2016.07.001.
- Ribatti D, Crivellato E, Vacca A. Miller's seminal studies on the role of thymus in immunity. Clin Exp Immunol. 2006 Jun;144(3):371-375. doi: 10.1111/j.1365-2249.2006.03060.x.
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318-1321. doi: 10.1126/science.3489291.
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):720-724. doi: 10.1073/pnas.90.2.720.
- Hu KJ, Yin ETS, Hu YX, Huang H. Combination of CRISPR/Cas9 System and CAR-T Cell Therapy: A New Era for Refractory and Relapsed Hematological Malignancies. Curr Med Sci. 2021 Jun;41(3):420-430. doi: 10.1007/s11596-021-2391-5.
- David RM, Doherty AT. Viral Vectors: The Road to Reducing Genotoxicity. Toxicol Sci. 2017 Feb;155(2):315-325. doi: 10.1093/toxsci/kfw220.
- Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019 May;18(5):358-378. doi: 10.1038/s41573-019-0012-9.
- Hajitou A, Lev DC, Hannay JA, Korchin B, Staquicini FI, Soghomonyan S, et al. A preclinical model for predicting drug response in soft-tissue sarcoma with targeted AAVP molecular imaging. Proc Natl Acad Sci U S A. 2008 Mar 18;105(11):4471-4476. doi: 10.1073/pnas.0712184105.
- Hajitou A, Rangel R, Trepel M, Soghomonyan S, Gelovani JG, Alauddin MM, et al. Design and construction of targeted AAVP vectors for mammalian cell transduction. Nat Protoc. 2007;2(3):523-531. doi: 10.1038/nprot.2007.51.
- Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC 3rd, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006 Apr 21;125(2):385-398. doi: 10.1016/j.cell.2006.02.042.
- Pranjol MZ, Hajitou A. Bacteriophage-derived vectors for targeted cancer gene therapy. Viruses. 2015 Jan 19;7(1):268-284. doi: 10.3390/v7010268.
- Lang LH. FDA approves use of bacteriophages to be added to meat and poultry products. Gastroenterology. 2006 Nov;131(5):1370. doi: 10.1053/j.gastro.2006.10.012.
- Atterbury RJ. Bacteriophage biocontrol in animals and meat products. Microb Biotechnol. 2009 Nov;2(6):601-612. doi: 10.1111/j.1751-7915.2009.00089.x.
- Zhang Y, Yu LC. Microinjection as a tool of mechanical delivery. Curr Opin Biotechnol. 2008 Oct;19(5):506-510. doi: 10.1016/j.copbio.2008.07.005.
- Stevenson DJ, Gunn-Moore FJ, Campbell P, Dholakia K. Single cell optical transfection. J R Soc Interface. 2010 Jun 6;7(47):863-871. doi: 10.1098/rsif.2009.0463.
- Hewapathirane DS, Haas K. Single cell electroporation in vivo within the intact developing brain. J Vis Exp. 2008 Jul 11;(17):705. doi: 10.3791/705.
- Christou P, McCabe DE, Swain WF. Stable Transformation of Soybean Callus by DNA-Coated Gold Particles. Plant Physiol. 1988 Jul;87(3):671-674. doi: 10.1104/pp.87.3.671.
- Klein RM, Wolf ED, Wu R, Sanford JC. High-velocity microprojectiles for delivering nucleic acids into living cells. 1987. Biotechnology. 1992;24:384-386.
- Lin MT, Pulkkinen L, Uitto J, Yoon K. The gene gun: current applications in cutaneous gene therapy. Int J Dermatol. 2000 Mar;39(3):161-170. doi: 10.1046/j.1365-4362.2000.00925.x.
- Yang CH, Shen SC, Lee JC, Wu PC, Hsueh SF, Lu CY, et al. Seeing the gene therapy: application of gene gun technique to transfect and decolour pigmented rat skin with human agouti signalling protein cDNA. Gene Ther. 2004 Jul;11(13):1033-1039. doi: 10.1038/sj.gt.3302264.
- Jiao S, Cheng L, Wolff JA, Yang NS. Particle bombardment-mediated gene transfer and expression in rat brain tissues. Biotechnology (N Y). 1993 Apr;11(4):497-502. doi: 10.1038/nbt0493-497.
- O'Brien JA, Holt M, Whiteside G, Lummis SC, Hastings MH. Modifications to the hand-held Gene Gun: improvements for in vitro biolistic transfection of organotypic neuronal tissue. J Neurosci Methods. 2001 Nov 15;112(1):57-64. doi: 10.1016/s0165-0270(01)00457-5.
- Wirth MJ, Wahle P. Biolistic transfection of organotypic cultures of rat visual cortex using a handheld device. J Neurosci Methods. 2003 May 30;125(1-2):45-54. doi: 10.1016/s0165-0270(03)00024-4.
- Monjezi R, Miskey C, Gogishvili T, Schleef M, Schmeer M, Einsele H, Ivics Z, Hudecek M. Enhanced CAR T-cell engineering using non-viral Sleeping Beauty transposition from minicircle vectors. Leukemia. 2017 Jan;31(1):186-194. doi: 10.1038/leu.2016.180.
- Shi J, Ma Y, Zhu J, Chen Y, Sun Y, Yao Y, Yang Z, Xie J. A Review on Electroporation-Based Intracellular Delivery. Molecules. 2018 Nov 21;23(11):3044. doi: 10.3390/molecules23113044.
- Zhang Z, Qiu S, Zhang X, Chen W. Optimized DNA electroporation for primary human T cell engineering. BMC Biotechnol. 2018 Jan 30;18(1):4. doi: 10.1186/s12896-018-0419-0.
- Pagant S, Liberatore RA. In Vivo Electroporation of Plasmid DNA: A Promising Strategy for Rapid, Inexpensive, and Flexible Delivery of Anti-Viral Monoclonal Antibodies. Pharmaceutics. 2021 Nov 6;13(11):1882. doi: 10.3390/pharmaceutics13111882.
- Heller LC, Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr Gene Ther. 2010 Aug;10(4):312-317. doi: 10.2174/156652310791823489.
- Byagathvalli G, Sinha S, Zhang Y, Styczynski MP, Standeven J, Bhamla MS. ElectroPen: An ultra-low-cost, electricity-free, portable electroporator. PLoS Biol. 2020 Jan 10;18(1):e3000589. doi: 10.1371/journal.pbio.3000589.
- Kwon M, Firestein BL. DNA transfection: calcium phosphate method. Methods Mol Biol. 2013;1018:107-110. doi: 10.1007/978-1-62703-444-9_10.
- Gulick T. Transfection using DEAE-dextran. Curr Protoc Cell Biol. 2003 Aug;Chapter 20:Unit 20.4. doi: 10.1002/0471143030.cb2004s19.
- Sariyer IK. Transfection of neuronal cultures. Methods Mol Biol. 2013;1078:133-139. doi: 10.1007/978-1-62703-640-5_11.
- Keller AA, Maeß MB, Schnoor M, Scheiding B, Lorkowski S. Transfecting Macrophages. Methods Mol Biol. 2018;1784:187-195. doi: 10.1007/978-1-4939-7837-3_18.
- Maeß MB, Wittig B, Lorkowski S. Highly efficient transfection of human THP-1 macrophages by nucleofection. J Vis Exp. 2014 Sep 2;(91):e51960. doi: 10.3791/51960.
- Maeß MB, Keller AA, Rennert K, Mosig A, Lorkowski S. Optimization of the transfection of human THP-1 macrophages by application of Nunc UpCell technology. Anal Biochem. 2015 Jun 15;479:40-42. doi: 10.1016/j.ab.2014.12.023.
- Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008 Jan;214(2):161-178. doi: 10.1002/path.2284.
- Oh S, Kessler JA. Design, Assembly, Production, and Transfection of Synthetic Modified mRNA. Methods. 2018 Jan 15;133:29-43. doi: 10.1016/j.ymeth.2017.10.008.
- Weissman D, Karikó K. mRNA: Fulfilling the Promise of Gene Therapy. Mol Ther. 2015 Sep;23(9):1416-1417. doi: 10.1038/mt.2015.138.
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014 Oct;13(10):759-780. doi: 10.1038/nrd4278.
- Zou S, Scarfo K, Nantz MH, Hecker JG. Lipid-mediated delivery of RNA is more efficient than delivery of DNA in non-dividing cells. Int J Pharm. 2010 Apr 15;389(1-2):232-243. doi: 10.1016/j.ijpharm.2010.01.019.
- Youn H, Chung JK. Modified mRNA as an alternative to plasmid DNA (pDNA) for transcript replacement and vaccination therapy. Expert Opin Biol Ther. 2015;15(9):1337-1348. doi: 10.1517/14712598.2015.1057563.
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010 Nov 5;7(5):618-630. doi: 10.1016/j.stem.2010.08.012.
- Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019 May;94(S1):S3-S9. doi: 10.1002/ajh.25418.
- Charkhchi P, Cybulski C, Gronwald J, Wong FO, Narod SA, Akbari MR. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers (Basel). 2020 Dec 11;12(12):3730. doi: 10.3390/cancers12123730.
- Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015 Feb;3(2):125-135. doi: 10.1158/2326-6066.CIR-14-0127.
- Harris DT, Kranz DM. Adoptive T Cell Therapies: A Comparison of T Cell Receptors and Chimeric Antigen Receptors. Trends Pharmacol Sci. 2016 Mar;37(3):220-230. doi: 10.1016/j.tips.2015.11.004.
- Minutolo NG, Hollander EE, Powell DJ Jr. The Emergence of Universal Immune Receptor T Cell Therapy for Cancer. Front Oncol. 2019 Mar 26;9:176. doi: 10.3389/fonc.2019.00176.
- Strohl WR, Naso M. Bispecific T-Cell Redirection versus Chimeric Antigen Receptor (CAR)-T Cells as Approaches to Kill Cancer Cells. Antibodies (Basel). 2019 Jul 3;8(3):41. doi: 10.3390/antib8030041.
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10024-10028. doi: 10.1073/pnas.86.24.10024.
- Zhao L, Cao YJ. Engineered T Cell Therapy for Cancer in the Clinic. Front Immunol. 2019 Oct 11;10:2250. doi: 10.3389/fimmu.2019.02250.
- Bagley SJ, O'Rourke DM. Clinical investigation of CAR T cells for solid tumors: Lessons learned and future directions. Pharmacol Ther. 2020 Jan;205:107419. doi: 10.1016/j.pharmthera.2019.107419.
- Pegram HJ, Park JH, Brentjens RJ. CD28z CARs and armored CARs. Cancer J. 2014 Mar-Apr;20(2):127-133. doi: 10.1097/PPO.0000000000000034.
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011 Aug 10;3(95):95ra73. doi: 10.1126/scitranslmed.3002842.
- Cheadle EJ, Gornall H, Baldan V, Hanson V, Hawkins RE, Gilham DE. CAR T cells: driving the road from the laboratory to the clinic. Immunol Rev. January 2014; 257(1):91–106. https://doi.org/10.1111/IMR.12126.
- Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. August 1, 2015; 15(8):1145–1154. https://doi.org/10.1517/14712598.2015.1046430.
- Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019 Mar;34:45-55. doi: 10.1016/j.blre.2018.11.002.
- Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. March 2014; 20(2):119–122. doi:10.1097/PPO.0000000000000035.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. October 16, 2014; 371(16):1507–17. doi: 10.1056/NEJMOA1407222.
- Hunter BD, Jacobson CA. CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions. J Natl Cancer Inst. 2019 Jul 1;111(7):646-654. doi: 10.1093/jnci/djz017. PMID: 30753567.
- Graham C, Hewitson R, Pagliuca A, Benjamin R. Cancer immunotherapy with CAR-T cells - behold the future. Clin Med (Lond). 2018 Aug;18(4):324-328. doi: 10.7861/clinmedicine.18-4-324.
- Lyman GH, Nguyen A, Snyder S, Gitlin M, Chung KC. Economic Evaluation of Chimeric Antigen Receptor T-Cell Therapy by Site of Care Among Patients With Relapsed or Refractory Large B-Cell Lymphoma. JAMA Netw Open. 2020 Apr 1;3(4):e202072. doi: 10.1001/jamanetworkopen.2020.2072. Erratum in: JAMA Netw Open. 2020 Apr 1;3(4):e208117.
- Drent E, Themeli M, Poels R, de Jong-Korlaar R, Yuan H, de Bruijn J, et al. A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization. Mol Ther. 2017 Aug 2;25(8):1946-1958. doi: 10.1016/j.ymthe.2017.04.024.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018 Apr;17(4):261-279. doi: 10.1038/nrd.2017.243.
- Smits E, Ponsaerts P, Lenjou M, Nijs G, Van Bockstaele DR, Berneman ZN, Van Tendeloo VF. RNA-based gene transfer for adult stem cells and T cells. Leukemia. 2004 Nov;18(11):1898-1902. doi: 10.1038/sj.leu.2403463.
- Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019 Mar;18(3):175-196. doi: 10.1038/s41573-018-0006-z.
- Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther. 2011 Dec;22(12):1575-86. doi: 10.1089/hum.2011.070.
- Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010 Nov 15;70(22):9053-9061. doi: 10.1158/0008-5472.CAN-10-2880.
- Foster JB, Choudhari N, Perazzelli J, Storm J, Hofmann TJ, Jain P, et al. Purification of mRNA Encoding Chimeric Antigen Receptor Is Critical for Generation of a Robust T-Cell Response. Hum Gene Ther. 2019 Feb;30(2):168-178. doi: 10.1089/hum.2018.145.
- Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65:333-347. doi: 10.1146/annurev-med-060512-150254.
- Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014 Feb;2(2):112-120. doi: 10.1158/2326-6066.CIR-13-0170. Erratum in: Cancer Immunol Res. 2015 Feb;3(2):217.
- Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022 Jan 7;375(6576):91-96. doi: 10.1126/science.abm0594.


