Влияние микробиоты на терапию ингибиторами иммунных контрольных точек
Автор: Каминский В.В., Кудинова Е.А., Кулинич Т.М., Джикия Е.Л., Большакова О.Б., Горбаренко А.В., Боженко В.К.
Журнал: Вестник Российского научного центра рентгенорадиологии Минздрава России @vestnik-rncrr
Рубрика: Обзоры, лекции
Статья в выпуске: 4 т.22, 2022 года.
Бесплатный доступ
В последние годы активно изучаются новые подходы к иммунотерапии опухолей - использованию лекарственных средств, нацеленных на индукцию или усиление противоопухолевого иммунного ответа. Нацеленные на ко-ингибирующие иммунные контрольные точки моноклональные антитела продемонстрировали клиническую эффективность при терапии многих злокачественных новообразований. Однако существуют ограничения в использовании этих препаратов: возникновение нежелательных явлений, связанных с вовлечением иммунной системы, и затруднение прогноза результатов терапии. В последние годы микробиом микроокружения опухоли и желудочно-кишечного тракта, а также его метаболиты рассматриваются как предикторы и факторы, влияющие на иммунную терапию онкологических заболеваний. В нашем обзоре мы обсуждаем доклинические и клинические исследования с анализом микробного состава кишечника и микроокружения опухоли в качестве биомаркера ответа и токсичности иммунной терапии, способы влияния на микробный состав биотопов человека с целью модуляции иммунной терапии, определяем конкретных представителей микрофлоры в качестве благоприятных и неблагоприятныхфакторов в терапии ингибиторами иммунных контрольных точек, определяем ряд ограничений в исследовании микрофлоры и дальнейшие перспективы в этой области.
Микробиом, иммунотерапия, ингибиторы иммунных контрольных точек, ctla-4, pd-1, pd-l1
Короткий адрес: https://sciup.org/149142256
IDR: 149142256
Текст научной статьи Влияние микробиоты на терапию ингибиторами иммунных контрольных точек
Federation, ORCID: 0000-0001-5702-6090
0000-0002-5530-0591
Federation, ORCID: 0000-0003-2331-5753
Federation, ORCID: 0000-0001-8369-2011
Scientific Center of Roentgenoradiology” of the Ministry of Healthcare of the Russian Federation, ORCID: 0000-0001-8382-3579
Gorbarenko A.V. – Junior Researcher of the Laboratory of Cell and Gene Therapy of the FSBI “Russian Scientific Center of Roentgenoradiology” of the Ministry of Healthcare of the Russian Federation, ORCID: 0000-0002-6355-7303
Bozhenko V.K. – Doctor of Medical Sciences, Professor, Head of the Department of Molecular Biology and Experimental Tumor Therapy of the FSBI “Russian Scientific Center of Roentgenoradiology” of the Ministry of Healthcare of the Russian Federation, ORCID: 0000-0001-8351-8152
В последние годы активно изучаются новые подходы к иммунотерапии опухолей — использованию лекарственных средств, нацеленных на индукцию или усиление противоопухолевого иммунного ответа [8,42,73]. Современные исследования в области иммунотерапии опухолей чрезвычайно обширны и наряду с изучением традиционных средств, таких как вакцины и противоопухолевые антитела, рассматривают агонисты Toll-подобных рецепторов (TLR), ингибиторы иммунных контрольных точек (ИКТ) и адоптивную клеточную терапию (ACT) [42]. Иммунологические контрольные точки – система ингибиторных механизмов, которые регулируют иммунный ответ и препятствуют запуску аутоиммунных процессов, а также моделируют его, уменьшая вызванные иммунными клетками повреждения в органах и тканях [1]. Воздействуя на контрольные точки, опухолевые клетки блокируют активацию опухоль-специфических лимфоцитов, приобретая при этом устойчивость к действию иммунной системы. Противоопухолевый ответ может быть восстановлен препаратами, подавляющими лиганд-рецепторное взаимодействие и деактивирующими ИКТ. Подробно механизмы действия препаратов ИКТ описаны в литературе отечественными и зарубежными авторами [4,6,36]. Моноклональные антитела, нацеленные на ко-ингибирующие иммунные контрольные точки, продемонстрировали клиническую эффективность при терапии таких злокачественных новообразований как меланома, немелкоклеточный рак легкого, почечно-клеточный рак, рак мочевого пузыря, плоскоклеточный рак головы и шеи, колоректальный рак с высокой степенью микросателлитной нестабильности, карцинома Меркеля и лимфома Ходжкина и тем самым изменили клиническую практику в онкологии [10,27,36]. Последнее десятилетие наблюдается беспрецедентный прогресс в иммунотерапии рака, и на сегодняшний день наиболее широко используются иммунотерапевтические агенты, блокирующие антитела, нацеленные на иммунные ингибирующие рецепторы, такие как CTLA-4, PD-1 и PD-L1. К ним относятся препараты ИКТ (пембролизумаб и ниволумаб), нацеленные на рецептор запрограммированной клеточной смерти 1 (PD-1), лиганд рецептора запрограммированной клеточной смерти 1 (PD-L1) (атезолизумаб, авелумаб, дурвалумаб и цемиплимаб) и цитотоксический Т-лимфоцит-ассоциированный антиген 4 (CTLA-4) (ипилимумаб, дурвалумаб и тремелимумаб), а также комбинированные препараты, ингибирующие CTLA-4 и PD-1 (ипилимумаб – ниволумаб) [63]. Хотя антитела против этих молекул уже являются одобренными в ряде стран препаратами для лечения различных типов рака, многочисленные антитела и небольшие молекулы, нацеленные на другие иммунные контрольные точки, такие как LAG3, TIGIT, TIM3, B7H3, CD39, CD73, рецептор аденозина A2A и CD47, находятся в стадии клинической разработки. В многочисленных клинических исследованиях эти препараты демонстрируют значительные результаты в повышении общей выживаемости пациентов и выживаемости без прогрессирования [32,54,72]. Так, при метастатической меланоме комбинированная блокада CTLA-4 и PD-1 позволила достичь пятилетней общей выживаемости, превышающей 50% [40]. При метастатической почечно-клеточной карциноме та же комбинация была связана с общей трехлетней выживаемостью, превысившей 60% у пациентов, получавших лечение ИКТ [51,52]. При этом терапия ИКТ зачастую сопровождается возникновением нежелательных явлений, связанных с вовлечением иммунной системы, связанных с аутоиммунными реакциями, которые сложно прогнозировать, диагностировать и лечить. В случае метастатической меланомы добавление антител CTLA-4 к блокаде PD-1 вызывает постепенное увеличение выживаемости, но при этом более чем в два раза возрастает частота серьезных нежелательных явлений, связанных с вовлечением иммунной системы [33]. Было предложено множество кандидатов на роль предикторов нежелательных явлений (исходная лимфопения и эозинофилия, Т- и В-клеточный состав, циркулирующий IL-17 и состав микробиоты кишечника и микроокружения опухоли), но лишь немногие из них были проспективно подтверждены.
Так, в мета-анализе Wang с соавторами сообщается, что показатель летальности у пациентов, получавших лечение с использованием монотерапии и комбинации ИКТ составляет 0,36 % – 1,23 % [70] . При возникновении осложнений, таких как иммунный миокардит, уровень смертности пациентов достигает 50% [47] . Более того, сообщается, что иммунотерапия ускоряет прогрессирование опухоли у значительной части пациентов в диапазоне от 4% до 29%, что вызывает опасения относительно ее дальнейшего клинического применения [25 ,56] . Также отмечены поздние рецидивы при более длительном наблюдении за участниками клинических исследований, что свидетельствует о возникновении приобретенной устойчивости [36] .
Роль микробиоты в онкологии достаточно широко освещена отечественными и зарубежными учеными в обзорах последних лет [5, 7 ,59] . Исследования микробного метагенома (совокупного генома) и метаболома (совокупности всех метаболитов, являющихся конечным продуктом обмена веществ в клетке) подчеркнули двойную роль желудочно-кишечной микробиоты в профилактике злокачественных новообразований, канцерогенезе и противоопухолевой терапии: микробиота кишечника может быть либо подавляющей опухоль, либо онкогенной [26 ,39 ,69] . При этом развитие онкологического заболевания может изменить микробиоту, и, в свою очередь, изменения микробиоты могут повлиять на течение онкологического заболевания. Хотя эта связь изучается давно, охарактеризована она лишь частично. В этом обзоре мы сосредоточимся на определении влияния микробиоты на терапию препаратами, ингибирующими CTLA-4, PD-1 и PD-L1 в онкологии.
Пути иммунных контрольных точек и механизм действия анти-CTLA-4 и анти-PD1 антител
CTLA-4 (cytotoxic T-lymphocyte-associated protein 4; CD152) клеточный рецептор, является ингибитором активации Т-клеток. CTLA-4, экспрессирующийся на поверхности CD4+ и CD8+ T-клеток, связывается с лигандами CD80 и CD86 на антигенпрезентирующих клетках (АПК), тем самым блокируя костимуляторный сигнал, что приводит к подавлению активации наивных клеток и T-клеток памяти. Ключевым является процесс, когда белок осуществляет транс-эндоцитоз, связывая молекулы лигандов на поверхности АПК и направляя их внутрь Т-клетки, в лизосомально-эндосомальный компартмент, где они подвергаются протеолизу (Рис. 1А) [3]. Помимо повышенной экспрессии на поверхности активированных Т-клеток, CTLA-4 также конститутивно экспрессируется на поверхности регуляторных Т-клеток (Т-супрессоры), которые контролируют силу и продолжительность иммунного ответа, посредством регуляции функции Т-эффекторных клеток (Т-киллеров и Т-хелперов) [30]. PD-1 (Programmed cell death 1; CD279) также является частью суперсемейства иммуноглобулинов. Он регулирует иммунные процессы, подавая ингибирующие сигналы при связывании со своими лигандами (Programmed death ligands 1/2; PD-L1/2) [17,41]. PD-1 экспрессируется на CD 4+ и CD 8+T-клетках, B-, NK, NKT-клетках и активированных моноцитах периферических тканей [3,17,41]. Подробные механизмы влияния иммунных контрольных точек и анти-CTLA-4 и анти-PD1 антител изображены на рисунке 1.
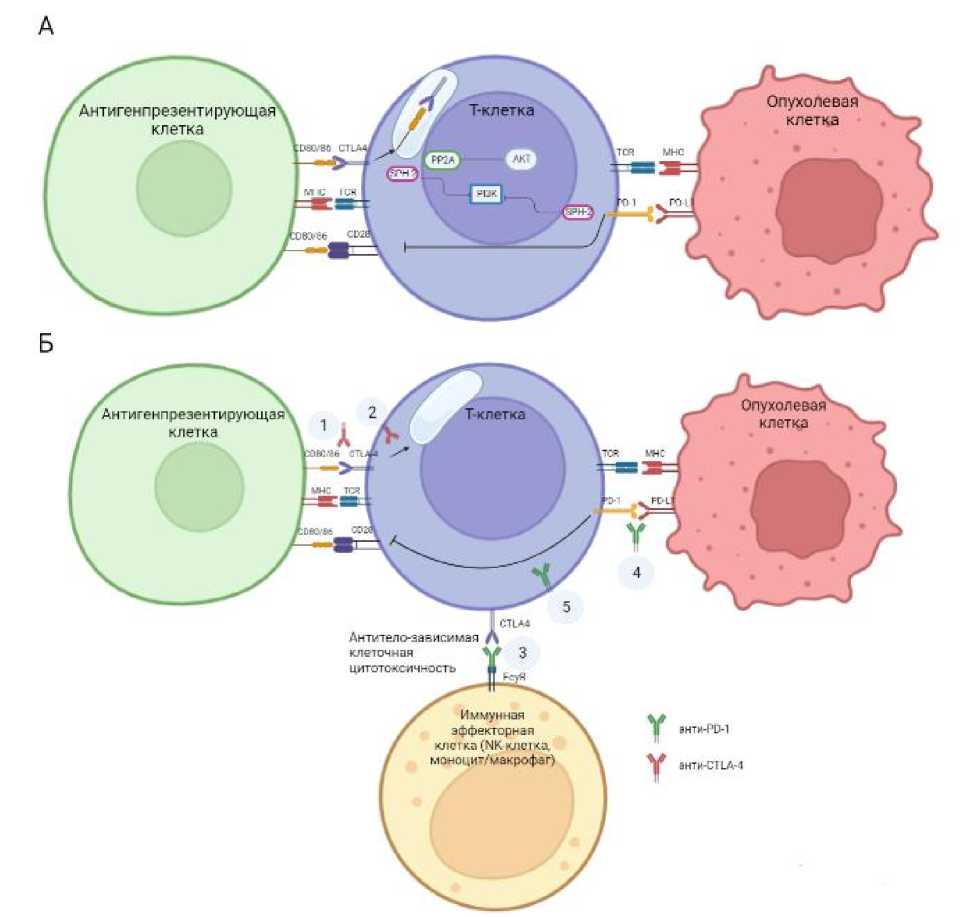
Рис. 1. Механизмы влияния иммунных контрольных точек и анти-CTLA-4 и анти-PDl антител/ (А) Пути иммунных контрольных точек и (Б) Механизм действия анти-CTLA-4 и анти-PDl антител.
Пути CTLA-4 и PD-1 негативно регулируют активацию T-клеток. Для активации T-клеток требуются 2 сигнала: первый - от взаимодействия Т-клеточного рецептора (TCR) с антигеном, представленным в молекулах главного комплекса гистосовместимости, второй -от костимуляторных рецепторов (наиболее важным из которых является молекула CD28) при взаимодействии с лигандами CD80/86 [44]. Ингибиторный рецептор CTLA-4 представляет собой конкурентный гомолог CD28, который связывается с теми же лигандами (CD80/86), что и CD28, но с большей аффинностью, блокируя второй сигнал, что препятствует активации Т-клеток [57,62,66]. CTLA-4 связывает лиганды CD80/86, осуществляет их трансэндоцитоз с последующим протеолизом внутри клетки. Эти ингибирующие сигналы в конечном итоге переводят Т-клетку в состояние покоя. Ингибиторный рецептор PD-1 взаимодействует со своим лигандом PD-L1/PD-L2, негативно регулируя активацию Т-клеток. CD28 также является второй мишенью для PD-1 и точкой пересечения двух сигнальных путей. Экспрессия как CTLA-4, так и PD-1 повышается при активации TCR. Внутриклеточная передача сигналов для обоих путей опосредуется фосфатазой с доменом гомологии Srс2 (SHP2), ингибирующей передачу сигналов PI3K ниже по пути [51]. CTLA-4, кроме того, взаимодействует с серин/треонинфосфатазой PP2A, которая дефосфорилирует киназу AKT, дополнительно ингибируя этот сигнальный путь [51,62].
Механизм действия анти-CTLA-4 и анти-PDl антител: (1) Ahtu-CTLA-4 восстанавливает активацию T-клеток путем ингибирования взаимодействия между CTLA-4 и CD80/CD86 на (АПК). (2) Ahtu-CTLA-4 антитела могут ингибировать трансэндоцитоз лигандов CD28 CD80/86, опосредованный через CTLA-4. (3) ипилимумаб (IgGl-антитело против CTLA-4) может взаимодействовать с гамма FcyR рецепторами на иммунных эффекторных клетках (NK-клетках, моноцитах/макрофагах) через свой Fc-участок, что приводит к антитело-зависимой клеточной цитотоксичности и истощению некоторых высокоактивных CTLA-4-экспрессирующих T-клеток (например, Т-супрессоров). (4) Анти-PD-1 антитела восстанавливают активацию Т-клеток путем ингибирования взаимодействия между PD-1 на Т-клетках и лигандом PD-L1/PD-L2, экспрессирующимся на различных иммунных и опухолевых клетках. (5) Анти-PD-l восстанавливает активацию Т-клеток за счет взаимодействия между PD-1 и CD28 в точке схождения двух путей.
Микробиота и анти-CTLA-4 терапия
В статье Vetizou с соавторами показано, что противоопухолевые эффекты блокады CTLA-4 зависят от различных видов Bacteroides. У мышей и пациентов ответы Т-клеток, специфичные для B. thetaiotaomicron или B. fragilis, были связаны с эффективностью блокады CTLA-4. Опухоли у мышей, получавших антибиотики, или у безмикробных мышей не реагировали на блокаду CTLA. Этот дефект был устранен введением B. fragilis через желудочный зонд, иммунизацией полисахаридами B. fragilis или адоптивным переносом специфичных для B. fragilis Т-клеток. Таким образом, исследователи показали, что состав микробиоты влияет на IL-12 зависимые иммунные ответы T-хелперов 1, которые усиливают эффекты анти-CTLA-4 терапии у мышей и пациентов. При этом сохраняется целостность кишечника и снижаются гистопатологические признаки колита, который уменьшает эффективность ИКТ [68]. Это исследование показывает важную роль Bacteroidales в иммуностимулирующих эффектах блокады CTLA-4.
Влияние микробиоты на снижение риска возникновения колита, связанного с терапией ипилимумабом также показано в проспективном исследовании пациентов с метастатической меланомой Dubin с соавторами. Проанализировав микробный пейзаж кишечника до и после начала анти-CTLA-4 терапии исследователи обнаружили, что повышенное содержание бактерий, принадлежащих к порядку Bacteroidetes , коррелирует с устойчивостью к развитию колита. Метагеномный анализ образцов фекалий пациентов показал большую представленность модулей бактериальной транспортной системы полиаминов, биосинтеза витаминов рибофлавина (витамин В2), пантотената (витамин В5) и тиамина (витамин В1) у пациентов без колита, подчеркивая роль бактерий в препятствии возникновения этого осложнения [23] .
В экспериментах Chaput с соавторами выявлено, что пациенты с метастатической меланомой, чья исходная кишечная микробиота была обогащена представителями Faecalibacterium и Firmicutes , имели более длительную общую выживаемость и статистически достоверную выживаемость без прогрессирования, низкую долю регуляторных Т-клеток периферической крови и долгосрочный клинический эффект ипилимумаба, по сравнению с пациентами с микрофлорой, богатой Bacteroides , при этом сам препарат не вызывал существенных изменений в составе микробиома кишечника пациентов.
Ипилимумаб приводил к увеличению уровня индуцируемого костимулятора на CD4+ Т- клетках и уровня CD25 в сыворотке крови у пациентов, чья микробиота до начала терапии была обогащена бактериями рода Faecalibacterium и другими представителями Firmicutes [14]. Таким образом, различный исходный состав микробиоты кишечника во многом может определять клинический ответ на терапию ипилимумабом.
Микробиота и комбинированная терапия
Frankel с соавторами провели проспективное исследование влияния микробиоты и метаболома кишечника человека на результаты лечения ИКТ у пациентов с метастатической меланомой, получавших комбинированную терапию анти-CTLA-4 и анти-PD1 (ипилимумаб, ниволумаб, ипилимумаб плюс ниволумаб), и анти-PD1 терапию (пембролизумаб). У пациентов, ответивших на комбинированную терапию (респондентов), микробиом кишечника был обогащен Faecalibacterium prausnitzii, Bacteroides thetaiotamicron и Holdemania filiformis. У пациентов, ответивших на анти-PD1 терапию, микрофлора была богата Dorea formicogenerans. У пациентов, ответивших на все виды терапии, в большом количестве были обнаружены Bacteroides caccae и Streptococcus parasanguinis [24]. В попытке получить функциональное представление об изменениях в микробиоме кишечника исследователи использовали анализ метагеномных функциональных путей. Интересно, что среди оцениваемых 1901 метаболитов наиболее выраженная корреляция с ответом на терапию была обнаружена у пациентов с высоким уровнем содержания ксенобиотика анакардовой кислоты, которая не является бактериальным метаболитом и, вероятно, содержалась в пище [24]. Известно, что анакардовые кислоты оказывают стимулирующее влияние на нейтрофилы и макрофаги, что может усиливать рекрутирование Т-клеток в метастазы опухоли и, следовательно, усиливать ИКТ [34]. Анакардовые кислоты продемонстрировали противоопухолевый эффект на нескольких доклинических моделях [31]. Дальнейшие исследования микробного пейзажа и метаболических путей могут позволить выявить направление причинно-следственной связи и определить предикторы эффективности терапии опухолей.
Исследования, проведенные Peters с соавторами , также подчеркивают влияние микробного разнообразия на благоприятные исходы терапии пациентов с меланомой анти-PD-1, анти- CTLA-4 и комбинированными иммунопрепаратами. Авторами были проведены метагеномные исследования кишечного содержимого пациентов с определением микробного разнообразия и метаболических путей. Они определили, что в группе с более короткой выживаемостью без прогрессии (ВБП) были пациенты с преобладанием видов Bacteroides ovatus , Bacteroides dorei , Bacteroides massiliensis , Ruminococcus gnavus и Blautia producta . А в группе с более длительной ВБП были пациенты с Faecalibacterium prausnitzii , Coprococcus eutactus , Prevotella stercorea , Streptococcus sanguinis , Streptococcus anginosus и Lachnospiraceae . Метагеномные функции (метатранскриптомная экспрессия), коррелирующие с ВБП, включали деградацию L-рамнозы, биосинтез гуанозиновых нуклеотидов и биосинтез витамина В [53] .
В исследовании Coutzac с соавторами анализ микробной 16S рРНК у пациентов с ММ, получавших лечение ипилимумабом, установлена связь наличия бактерий рода Faecalibacterium и Gemminger с долгосрочной выживаемостью без прогрессирования (>6 месяцев). Высокая относительная численность Faecalibacterium на исходном уровне была связана с общей выживаемостью (ОВ) более 18 месяцев. Кишечная микробиота способна влиять на иммунные реакции организма человека при помощи своих метаболитов, таких как короткоцепочечные жирные кислоты (ацетат, пропионат и бутират), производные индола, полиамины и другие, которые вырабатываются в больших количествах в толстой кишке в результате бактериальной ферментации пищевых волокон [65]. Одними из производителей короткоцепочечных жирных кислот являются бактерии рода Faecalibacterium и другие представители Firmicutes. Результаты определения Coutzac с соавторами концентрации короткоцепочечных жирных кислот в сыворотке крови показали, что низкий исходный уровень бутирата, так и низкий исходный уровень пропионата были связаны с более длительной ВБП. Это позволило предположить, что концентрации бутирата и пропионата в сыворотке могут представлять собой косвенный системный маркер состава микробиоты, связанный с клиническими исходами у пациентов, получавших ипилимумаб. Это предположение подтвердили на лабораторных животных, определив, что бутират снижает противоопухолевую эффективность анти-CTLA-4 у мышей, снижая активацию через рецепторы CD80/86 на дендритных клетках и рецептора индуцируемого костимулятора на Т-клетках (Inducible T-cell costimulator; ICOS), накопление опухолеспецифических Т-клеток и Т-клеток памяти. У пациентов, у которых были отмечены высокие уровни бутирата в крови, после воздействия ипилимумаба наблюдалось умеренное накопление клеток памяти, ICOS + CD4 + T-клеток и уровня IL-2 [15].
В ходе недавних исследований Andrews с соавторами определили преобладающие виды у пациентов, ответивших на терапию ( Bacteroides stercoris , Parabacteroides distasonis и Fournierella massiliensis ) и пациентов, не ответивших на комбинированную терапию ИКТ (включали, среди прочего, Klebsiella aerogenes и Lactobacillus rogosae ). Также авторы отметили значительно более высокое содержание Bacteroides intestinalis у пациентов с нежелательными явлениями, связанными с вовлечением иммунной системы, и их связь с повышенной регуляцией IL-1β слизистой оболочки в образцах пациентов с колитом и экспериментах с животными моделями [9] .
Микробиота и анти-PD-1/PD-L1 терапия
В исследованиях Sivan с соавторами показано, что комменсальные бактерии рода Bifidobacterium стимулируют выработку дендритных клеток, приводящую к усилению поступления и накоплению CD8+ Т-клеток в микроокружении опухоли. Их пероральное введение мышам вызывало задержку роста опухоли в той же степени, что и терапия с использованием специфических антител PD-L1, а комбинированное лечение (введение Bifidobacterium и анти-PD-L1 терапия) почти полностью прекратило рост опухоли [64] .
Gopalakrishnan с соавторами отметили, что микробиомы полости рта и кишечника у пациентов с метастатической меланомой, получающих иммунотерапию анти-PD-1, демонстрируют высокое содержание бактерий Lactobacillales в полости рта и Bacteroidales в фекалиях. Авторы сравнили микрофлору пациентов, ответивших (R) и не ответивших (NR) на анти-PD-1 терапию. В кишечнике R превалирующими оказались представители Clostridiales / Ruminococcaceae (в основном за счет бактерий вида Faecalibacterium ), а бактерии класса Bacteroidales (за счет Bacteroides thetaiotaomicron , Escherichia coli и Anaerotruncus colihominis ) оказались более широко представленными в кишечнике у NR. В микрофлоре ротовой полости среди обеих групп различий не наблюдалось. Авторы оценили системный и противоопухолевый иммунный ответ и отметили у пациентов с «благоприятным» микробиомом кишечника (например, с высоким разнообразием и обилием Ruminococcaceae / Faecalibacterium ) усиленные системные и противоопухолевые иммунные реакции, опосредованные увеличением концентрации CD8+ рецепторов, и улучшенную функцию эффекторных Т-клеток на периферии и в микроокружении опухоли. Пациенты с «неблагоприятным» микробиомом кишечника (например, с низким разнообразием и высоким относительным обилием Bacteroidales ) демонстрировали нарушение системных и противоопухолевых иммунных ответов, ограниченную внутриопухолевую лимфоидную и миелоидную инфильтрацию, а также снижение числа CD8+ рецепторов [29] . Эти результаты подчеркивают терапевтический потенциал коррекции микробиома кишечника у пациентов, получающих ИКТ, и важность оценки микробного разнообразия у онкологических пациентов в ходе клинических испытаний.
В исследованиях Matson с соавторами было высказано предположение, что состав комменсальной микробиоты у пациентов коррелирует с терапевтической эффективностью моноклональных антител против PD-L1. Образцы кала были собраны и проанализированы с помощью различных молекулярно-генетических методов у 42 пациентов с метастатической меланомой до начала лечения. В результате проведенной терапии были определены 16
ответивших (R) и 26 не ответивших на терапию пациентов (NR). Бактериальные виды, более многочисленные у R, включали Bifidobacterium longum , Collinsella aerofaciens и Enterococcus faecium . Последующее проведение фекальной трансплантации мышам материала от R, привело к снижению роста опухоли, усилению Т-клеточного ответа и большей эффективности терапии анти-PD-L1 [45] .
В другом исследовании Routy с соавторами влияние кишечного микробиома на терапию анти-PD-1 препаратами изучалось у пациентов с немелкоклеточным раком легких, уротелиальной карциномой и почечно-клеточной карциномой. Авторы показали, что устойчивость к ИКТ может быть связана с составом кишечного микробиома. Антибиотики подавляли клинический эффект терапии ИКТ у пациентов с диссеминированным раком. Трансплантация фекальной микробиоты от больных раком, которые ответили на ИКТ, безмикробным мышам или мышам, получавшим антибиотики, улучшала противоопухолевые эффекты блокады PD-1, в то время как фекальная трансплантация от не ответивших на терапию пациентов не давала этого эффекта. Метагеномное исследование образцов кала пациентов выявило корреляцию между клиническим ответом на ИКТ и относительной численностью Akkermansia muciniphila . Пероральное введение A. muciniphila мышам, ранее подвергавшимся фекальной трансплантации кала пациентов, не отвечающих на терапию, повысило эффективность блокады PD-1 IL-12-зависимым образом за счет увеличения рекрутирования CCR9+CXCR3+CD4+ Т-лимфоцитов в микроокружении опухоли у мышей [55] .
Как упоминалось ранее, терапия ИКТ сопряжена с риском возникновения ряда осложнений, в частности с развитием ИКТ-ассоциированного колита [16]. Wang с соавторами сообщили о случаях успешно вылеченного ИКТ-ассоциированного колита трансплантацией фекальной микробиоты с восстановлением микробиома кишечника и относительным увеличением доли регуляторных Т-клеток в слизистой оболочке толстой кишки пациентов, получавших анти-CTLA-4 и комбинированную анти-CTLA-4 и анти-PD-1
терапию [71] .
Jin с коллегами в своем эксперименте изучили взаимосвязь между микробиомом кишечника и результатами терапии у пациентов с прогрессирующей немелкоклеточной карциномой легкого, которые получали терапию ингибиторами PD-1. Согласно результатам, пациенты, ответившие на лечение, обладали более высоким разнообразием и стабильным составом микробиома кишечника во время лечения и показали значительно более длительную выживаемость без прогрессирования. Секвенирование гена 16S рРНК показало, что Alistipes putredinis , Bifidobacterium longum и Prevotella copri были больше представлены у пациентов, ответивших на терапию, тогда как Ruminococcus-unclassified был обнаружен в основном у пациентов, не ответивших на лечение. Анализ системных иммунных ответов с помощью многоцветной проточной цитометрии показал, что пациенты с большим разнообразием микробиома в кишечнике имели более высокую представленность уникальных CD8+ Т-клеток памяти (GZMB+CD8+ Tm, Ki67+CD8+ Tm и CD8+ Tcm) и субпопуляций естественных киллеров в периферической крови в ответ на терапию анти-PD-1 [38] .
Zheng с соавторами исследовали образцы фекалий пациентов с гепатоцеллюлярной карциномой (ГЦК). У пациентов, не ответивших на лечение анти-PD-1, доля Proteobacteria увеличивалась с 3-й недели и стала доминирующей на 12-й неделе терапии. Увеличение доли Proteobacteria у NR объяснялось преобладанием Escherichia coli, в то время как наиболее заметными у пациентов, ответивших на терапию, были бактерии Klebsiella pneumoniae. Также в микрофлоре R преобладали бактерии Akkermansia muciniphila и Ruminococcaceae spp. Анализ функциональных генов и метаболических путей, таких как метаболизм углеводов и биосинтез метана, подтвердили потенциальную биоактивность видов, представленных у пациентов, ответивших на ИКТ [74]. Таким образом, микробиом кишечника влияет на ответ на анти-PD-1 терапию у пациентов с ГЦК. Авторы считают, что динамические характеристики микробиома кишечника могут быть использованы для раннего прогнозирования шестимесячных результатов анти-PD-1 иммунотерапии при ГЦК через 3–6 недель от начала лечения, что имеет решающее значение для мониторинга заболевания и принятия рациональных терапевтических решений.
В недавнем проспективном многоцентровом исследовании, основанном на профилировании микробиоты кишечника 338 пациентов с распространенным НМРЛ, получавших блокаду PD-1, также выявлена взаимосвязь наличия бактерии Akkemancia muciniphila у пациентов с увеличением частоты ответов на терапию ИКТ на 10 % [20] . Влияние Akkemancia muciniphila на ответ анти--PD-1 терапии было доказано на мышиных моделях и клеточных культурах. Это может позволить использовать эту бактерию в качестве биологического маркера эффективности анти-PD-1 терапии.
В настоящее время общепризнано, что кишечная микробиота восстанавливается через несколько недель после введения антибиотиков. Однако исследования Jernberg с коллегами показали, что даже кратковременное воздействие антибиотиков может иметь стойкое долгосрочное влияние на микробиоту кишечника человека, которое сохраняется до 2 лет после лечения [37]. Derosa с соавторами обследовали пациентов с прогрессирующей почечно-клеточной карциномой (ПКР) и немелкоклеточным раком легкого (НМРЛ), получавших моно- или комбинированную терапию ИКТ в сочетании или без предшествующей антибиотикотерапии (АБТ). У пациентов с почечно-клеточной карциномой терапия ИКТ в сочетании с АБТ приводила к повышенному риску первичного прогрессирующего заболевания в 75% случаев (против 22% у пациентов без АБТ), более короткой выживаемости без прогрессирования (медиана 1,9 против 7,4 месяцев у пациентов без АБТ) и более короткой общей выживаемости (медиана 17,3 против 30,6 месяцев у пациентов без АБТ). У пациентов с НМРЛ АБТ ассоциировалась с аналогичной частотой первичного прогрессирования заболевания (52% против 43% у пациентов без АБТ), но снижала выживаемость без прогрессирования (медиана 1,9 против 3,8 месяцев, P = 0,03) и общую выживаемость (медиана 7,9 против 24,6 месяцев, P <0,01). В многомерном анализе влияние АБП оставалось значительным для выживаемости без прогрессирования при ПКР и общей выживаемости при НМРЛ [21]. Таким образом, АБТ была связана с уменьшением клинической пользы от ИКТ при ПКР и НМРЛ. Влияние АБТ на состав кишечной микробиоты должно учитываться при выборе стратегии лечения для улучшения клинических исходов пациентов, которым проводится ИКТ.
Формирование эффективного противоопухолевого Т-клеточного ответа в решающей степени зависит от доступности для иммунных клеток питательных веществ, таких как аминокислота L-аргинин [28] . Следовательно, увеличение обычно низких концентраций L-аргинина в опухоли может значительно усиливать противоопухолевый ответ ингибиторов иммунных контрольных точек, таких как анти-PD-L1 антитела. Однако в настоящее время нет средств для локального повышения внутриопухолевого уровня L-аргинина. При этом микроокружение опухоли является обедненной питательными веществами средой [35] . Canale с коллегами ввели мышам генетически измененный штамм E. coli , способный синтезировать L-аргинин, что на фоне анти- PD-L1 терапии привело к формированию устойчивого долгосрочного Т-клеточного противоопухолевого иммунитета [13] .
Метаболом микробиома представляет собой конечные продукты микробного метаболизма, и его изучение может быть более важным для определения влияния на организм человека и эффективность иммунотерапии, в частности, чем определение микробного разнообразия или преобладания отдельных видов бактерий, связанных с тем или иным исходом лечения. Исследования Nomura с соавторами продемонстрировали, что концентрации короткоцепочечных жирных кислот (ацетат, пропионат, валерат и изовалерат) в кале могут быть связаны с увеличением эффективности анти-PD-1 терапии [50] . Таким образом, короткоцепочечные жирные кислоты могут быть связующим звеном между кишечной микробиотой и эффективностью PD-1.
Аналогичным образом Botticelli с соавторами оценили метаболом фекального микробиома у 11 пациентов с немелкоклеточным раком легкого, которым проводилось лечение ниволумабом [12] . Исследователи обнаружили, что у пациентов с ранним прогрессированием заболевания (прогрессирование заболевания в течение 3 месяцев после начала приема ниволумаба) метаболом микробиома характеризовался низким уровнем как короткоцепочечных жирных кислот (пропионовой, масляной, уксусной и валериановой кислот), так и аминокислот (лизина, изолейцина и глутаминовой кислоты), но высоким содержанием алканов, метилкетонов и p-крезола.
Baruch с коллегами была проведена I фаза клинического испытания (NCT03353402) для оценки безопасности и осуществимости трансплантации фекальной микробиоты (FMT) от доноров со стойким полным ответом и повторной анти-PD-1 иммунотерапии у 10 пациентов с резистентной к PD-1 метастатической меланомой. В результате проведенной терапии у ранее резистентных пациентов с рефрактерной метастатической меланомой, получен 1 полный и 2 частичных ответа [11] . В ходе клинических испытаний (NCT03341143) Davar с соавторами получили результаты, подтверждающие опыт Baruch с коллегами, что трансплантация фекальной микробиоты от пациентов, ответивших на анти-PD-1 терапию, изменила микробиом кишечника испытуемых и перепрограммировала микроокружение опухоли, что дало возможность преодолеть устойчивость к анти-PD-1 терапии прогрессирующей меланомы PD-1 у 6 из 15 пациентов. Микробиом пациентов, ответивших на терапию, характеризовался повышенным содержанием таксонов, которые связаны с ответом на анти-PD-1 терапию, а именно бактерий типа Firmicutes (семейства Lachnospiraceae и Ruminococcaceae ) и Actinobacteria (семейства Bifidobacteriaceae и Coriobacteriaceae). Наличие этих бактерий увеличивало активацию CD8+ Т-клеток и снижало частоту миелоидных клеток, экспрессирующих IL-8 [18] . Эти результаты подтверждают концепцию преодоления устойчивости к иммунотерапии путем моделирования микробиоты кишечника.
Обсуждение
Быстрый прогресс, достигнутый в области иммунотерапии контрольных точек, показал большой спектр проблем, связанных с токсичностью проводимого лечения и исследованием механизмов резистентности, характерной для ряда пациентов. Стандартные инструменты, используемые для оценки выбора лечения в эпоху химиотерапии и таргетной терапии, могут не подходить для новых иммунотерапевтических методов [52 ,60] . Осознание того, что микробиологический пейзаж кишечника человека и микроокружения опухоли может играть ключевую роль в современной иммунотерапии рака, заставляет исследователей искать точки воздействия на микробиоту с целью усиления эффективности терапии, снижения риска возникновения осложнений, возможности прогнозировать эффективность терапии и течение заболевания с помощью новых биомаркеров.
Хотя проведенные исследования демонстрируют влияние микрофлоры, ее метаболитов на эффективность ИКТ, четкой направленности причинно-следственной связи и механизмов этого влияния не установлено. Необходимо продолжить более масштабные и подробные лабораторные и клинические исследования, которые помогут заложить основу для оптимизации реакции организма хозяина на ИКТ и позволят оценивать, прогнозировать и улучшать терапию опухолей.
Остается спорным, насколько хорошо биомаркеры реакций (бактерии и их метаболиты) блокады CTLA-4, PD-1 и PD-L1 соотносятся между собой. Также в разработке находятся многочисленные стратегии нацеливания на другие контрольные точки, будь то блокада ингибирующих молекул или активация стимулирующих.
Наконец, становится все более очевидным, что такие факторы как общий иммунный статус хозяина, микробиом кишечника и микроокружение опухоли могут влиять на терапевтические реакции, и эти факторы необходимо учитывать в более целостном подходе к терапии злокачественных опухолей.
Назначение антибактериальных препаратов [62] и изменения в диете [63], которые не всегда фиксируются в экспериментах исследователей, также могут влиять на изменение состава микрофлоры пациентов. Будущие исследования в контексте ИКТ должны включать последовательный и детальный мониторинг микрофлоры в соответствии с изменениями в терапии и питании пациентов для получения достоверных результатов, тем более, что исследование микрофлоры кишечника представляет собой неинвазивную процедуру, которая в перспективе, позволит безболезненно оценить прогноз терапии. Характеристики динамических изменений микробиома кишечника могут обеспечить ранние прогнозы результатов иммунотерапии ИКТ, что имеет решающее значение для принятия рациональных терапевтических решений.
Биопленки являются основной формой существования бактерий в природе, и до 90% всех известных видов бактерий способны формировать биопленки, которые обнаруживаются в 80% случаев всех хронических инфекционных и воспалительных заболеваний человека [2] . Недавние обзоры научных публикаций демонстрируют роль биопленок в развитии онкозаболеваний [46 ,67] . Таким образом, исследование ее состава и факторов, способствующих ее образованию, также стоит учитывать при изучении микрофлоры и оценки ее влияния на течение терапии и исходы ИКТ.
Биотехнологические подходы в использовании генетически измененных штаммов в терапии представляют собой уникальные средства локальной метаболической модуляции микросреды опухоли с целью усиления ИКТ [13] . Данный подход представляется перспективным и требует дальнейшего развития для оценки в клинике.
Антибактериальные препараты оказывают влияние на течение и исходы ИКТ посредством изменения микробного пейзажа организма человека. Однако действие антибиотиков носит неспецифичный характер, что усложняет таргетное изменение микробиоты в сторону «благоприятных» для терапии ИКТ микроорганизмов. Эту проблему могла бы исправить специфическая деконтаминация отдельных «нежелательных» таксонов бактерий препаратами бактериофагов, эндолизинов и других целевых антибактериальных средств.
Пробиотики также оказывают влияние на терапию ИКТ. Исследования продемонстрировали положительное влияние представителей Bifidobacterium spp. на противораковое действие блокады PDL-1 [64] . Дальнейшие исследования должны быть направлены на поиск новых компонентов микробиоты, оказывающих положительное влияние на иммунотерапию.
Учитывая широкое применение ИКТ с расширением показаний для разных типов рака, ожидается, что будет увеличиваться рост развития нежелательных явлений, связанных с вовлечением иммунной системы. Модуляция микробиома кишечника посредством фекальной трансплантации микробиоты может стать терапевтической стратегией решения этой проблемы. Для оценки полезности этого подхода, а также для получения дополнительной информации о механизмах его влияния необходимы дальнейшие исследования.
Выводы
Будущее иммунотерапии рака может зависеть от использования ингибиторов контрольных точек в комплексе с другими методами лечения, где рычагом управления может стать воздействие на микробиом, направленное на микроокружение опухоли, микрофлору кишечника и бактериальные метаболиты. Сочетание исследований метагеномики, метатранскриптомики, метапротеомики и метаболомики может способствовать пониманию функциональной активности микробиома человека и обеспечить новую стратегию диагностики и лечения онкологических заболеваний.
Список литературы Влияние микробиоты на терапию ингибиторами иммунных контрольных точек
- Боголюбова А.В., Ефимов Г.А., Друцкая М.С. и др. Иммунотерапия опухолей, основанная на блокировке иммунологических контрольных «точек» («чекпойнтов»). Медицинская иммунология. 2015. Т. 17. № 5. С. 395-406. DOI: 10.15789/1563-0625-2015-5-395-406.
- Гостев В.В., Сидоренко С.В. Бактериальные биопленки и инфекции. Журнал инфектологии. 2014. Т. 2. № 3. С. 4-15. DOI: 10.22625/2072-6732-2010-2-3-4-15.
- Кадагидзе З.Г., Черткова А.И., Заботина Т.Н. и др. CTLA-4, PD-1/PD-L1 негативные регуляторы Т-клеточного иммунитета в терапии рака яичников. Онкогинекология. 2019. Т. 2. № 30. С. 4. DOI: 10.52313/22278710-2019-2-4.
- Мансорунов Д.Ж., Алимов А. А., Апанович Н. В. и др. Иммунотерапия рака желудка. Российский биотерапевтический журнал. 2019. Т. 18. № 4. С. 6-16. DOI: 10.17650/1726-9784-2019-18-4-06-16.
- Моисеенко Ф.В., Югай С.В., Волков Н.М. Микробиом и его роль в онкологии. Практическая онкология. 2020. Т. 21. № 1. С. 11-20. DOI: 10.31917/2101011.
- Набережнов Д.С., Морозов А.А., Фридман М.В. и др. Система pd-1/pd-l1 при иммунотерапии рака почки. Часть 1. Сигнальный путь pd-1/pd-l1, его роль в иммунной системе и иммунотерапии. Часть 1. Медицинский алфавит. 2018. Т. 2. № 29. С. 22-31.
- Сухина М. А., Лягина И. А., Сафин А. Л. и др. Роль кишечной микробиоты в колоректальном канцерогенезе (обзор литературы). Колопроктология. 2021. Т. 20. № 1. С. 68-76. DOI: 10.33878/2073-7556-2021-20-1-68-76.
- Abbott M., Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Seminars in oncology nursing. WB Saunders, 2019. V. 35. No. 5. Article ID 150923. DOI: 10.1016/j.soncn.2019.08.002.
- Andrews M.C., Duong C.P.M., Gopalakrishnan V., et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021. V. 27. No. 8. P. 1432-1441. DOI: 10.1038/s41591-021-01406-6.
- Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021. V. 16. P. 223-249. DOI: 10.1146/annurev-pathol-042020-042741.
- Baruch E.N., Youngster I., Ben-Betzalel G., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021. V. 371. No. 6529. P. 602-609. DOI: 10.1126/science.abb592.
- Botticelli A., Vernocchi P., Marini F., et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 2020. V. 18. No. 1. P. 1-10. DOI: 10.1186/s12967-020-02231-0.
- Canale F. P., Basso C., Antonini G., et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature. 2021. V. 598. No. 7882. P. 662-666 DOI: 10.1038/s41586-021-04003-2.
- Chaput N., Lepage P., Coutzac C., et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab.Ann Oncol. 2017. V. 28. No. 6. P. 1368-1379. DOI: 10.1093/annonc/mdx108.
- Coutzac C., Jouniaux J.M., Paci A., et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 2020. V. 11. No. 1. P. 1-13. DOI:10.1038/s41467-020-16079-x.
- Cramer P., Bresalier R.S. Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep. 2017. V. 19. No. 1. Article ID 3. DOI: 10.1007/s11894-017-0540-6.
- Dammeijer F., Gulijk M., Mulder E.E., et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell. 2020. V. 38. No. 5. P. 685-700. e8. DOI: 10.1016/j.ccell.2020.09.001.
- Davar D., Dzutsev A.K., McCulloch J.A., et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021. V. 371. No. 6529. P. 595-602. DOI: 10.1126/science.abf33.
- David L., Maurice C., Carmody R., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014. V. 505. No. 7484. P. 559-563. DOI: 10.1038/nature12820.
- Derosa L., Routy B., Thomas A.M., et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022. V. 28. No. 2. P. 315-324. DOI: 10.1038/s41591-021-01655-5.
- Derosa L., Hellmann M.D., Spaziano M., et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018. V. 29. No. 6. P. 1437-1444. DOI: 10.1093/annonc/mdy103.
- Dethlefsen L., Relman D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011. V. 108. Suppl. 1. P. 4554-4561. DOI: 10.1073/pnas.1000087107.
- Dubin K., Callahan M., Ren B., et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016. V. 7. No. 1. P. 1-8. DOI: 10.1038/ncomms10391.
- Frankel A.E., Coughlin L.A., Kim J., et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017. V. 19. No. 10. P. 848-855. DOI: 10.1016/j.neo.2017.08.004.
- Fuentes-Antrás J., Provencio M., Díaz-Rubio E. Hyperprogression as a distinct outcome after immunotherapy. Cancer Treat Rev. 2018. V. 70. P. 16-21. DOI: 10.1016/j.ctrv.2018.07.006.
- Gagnaire A., Nadel B., Raoult D., et al. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nat Rev Microbiol. 2017. V. 15. No. 2. P. 109-128. DOI: 10.1038/nrmicro.2016.171.
- Galluzzi L., Humeau J., Buqué A., et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020. V. 17. No. 12. P. 725-741. DOI: 10.1038/s41571-020-0413-z.
- Geiger R., Rieckmann J.C., Wolf T., et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016. V. 167. No. 3. P. 829-842. e13. DOI: 10.1016/j.cell.2016.09.031.
- Gopalakrishnan V., Spencer C.N, Nezi L., et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018. V. 359. No. 6371. P. 97-103. DOI: 10.1126/science.aan4236.
- Ha D., Tanaka A., Kibayashi T., et al. Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti–CTLA-4 antibody. Proc Natl Acad Sci U S A. 2019. V. 116. No. 2. P. 609-618. DOI: 10.1073/pnas.1812186116.
- Hemshekhar M., Santhosh M. S., Kemparaju K., et al. Emerging roles of anacardic acid and its derivatives: a pharmacological overview. Basic Clin Pharmacol Toxico. 2012. V. 110. No. 2. P. 122-132. DOI: 10.1111/j.1742-7843.2011.00833.x.
- Hodi F.S., O'Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010. V. 363. No. 8. P. 711-723. DOI: 10.1056/NEJMoa1003466.
- Hodi F.S., Chiarion-Sileni V., Gonzalez R., et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018. V. 19. No. 11. P. 1480-1492. DOI: 10.1016/S1470-2045(18)30700-9.
- Hollands A., Corriden R., Gysleret G., al. Natural product anacardic acid from cashew nut shells stimulates neutrophil extracellular trap production and bactericidal activity. J Bioll Chem. 2016. V. 291. No. 27. P. 13964-13973. DOI: 10.1074/jbc.M115.695866.
- Hou A.J., Chen L.C., Chen Y.Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. 2021. V. 20. No. 7. P. 531-550. DOI: 10.1038/s41573-021-00189-2.
- Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018. V. 118. No. 1. P. 9-16. DOI: 10.1038/bjc.2017.434.
- Jernberg C., Löfmark S., Edlund C., et al. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007. V 1. No. 1. P. 56-66. DOI: 10.1038/ismej.2007.3.
- Jin Y., Dong H., Xia L., et al. The diversity of gut microbiome is associated with favorable responses to anti–programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol. 2019. V. 14. No. 8. P. 1378-1389. DOI: 10.1016/j.jtho.2019.04.007.
- Knight R., Callewaert C., Marotz C., et al. The microbiome and human biology. Annu Rev Genomics Hum Genet. 2017. V. 18. P. 65-86. DOI: 10.1146/annurev-genom-083115-022438.
- Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019. V. 381. No. 16. P. 1535-1546. DOI: 10.1056/NEJMoa1910836.
- Latchman Y., Wood C., Chernova T., et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001. V. 2. No. 3. P. 261-268. DOI: 10.1038/85330.
- Lee L, Gupta M, Sahasranaman S. Immune Checkpoint inhibitors: An introduction to the next‐generation cancer immunotherapy. J Clini Pharmacol. 2016. V. 56. No. 2. P. 157-169. DOI: 10.1002/jcph.591.
- Li S.M. The Biological Function of SHP2 in Human Disease. Mol Biol (Mosk). 2016. V. 50. No. 1. P. 27-33. DOI: 10.7868/S0026898416010110.
- Linsley P.S., Brady W., Grosmaire L., et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991. V. 173. No. 3. P. 721-730. DOI: 10.1084/jem.173.3.721.
- Matson V., Fessler J., Bao R., et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018. V. 359. No. 6371. P. 104-108. DOI: 10.1126/science.aao3290.
- Mirzaei R., Mirzaei H., Alikhani M. Y., et al. Bacterial biofilm in colorectal cancer: What is the real mechanism of action? Microb Pathog. 2020. V. 142. Article ID 104052. DOI: 10.1016/j.micpath.2020.104052.
- Moslehi J.J., Salem J.E., Sosman J.A., et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018. V. 391. No. 10124. P. 933. DOI: 10.1016/S0140-6736(18)30533-6.
- Motzer R.J., Rini B.I., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019. V. 20. No. 10. P. 1370-1385. DOI: 10.1016/S1470-2045(19)30413-9.
- Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018. V. 378. No. 14. P. 1277-1290. DOI: 10.1056/NEJMoa1712126.
- Nomura M., Nagatomo R., Doi K., et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020. V. 3. No. 4. Article ID e202895. DOI: 10.1001/jamanetworkopen.2020.2895.
- Okazaki T., Maeda A., Nishimura H., et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001. V. 98. No. 24. P. 13866-13871. DOI: 10.1073/pnas.231486598.
- Pagès F., Mlecnik B., Marliot F., et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018. V. 391. No. 10135. P. 2128-2139. DOI: 10.1016/S0140-6736(18)30789-X.
- Peters B. A., Wilson M., Moran U., et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 2019. V. 11. No. 1. P. 1-14. DOI: 10.1186/s13073-019-0672-4.
- Robert C., Long G. V., Brady B., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015. V. 372. No. 4. P. 320-330. DOI: 10.1056/NEJMoa1412082.
- Routy B., Le Chateliere E., Derosa L., et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018. V. 359. No. 6371. P. 91-97. DOI: 10.1126/science.aan3706.
- Saâda-Bouzid E., Defaucheux C., Karabajakian A., et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017. V. 28. No. 7. P. 1605-1611. DOI: 10.1093/annonc/mdx178.
- Sadreddini S., Baradaran B., Aghebati-Maleki A., et al. Immune checkpoint blockade opens a new way to cancer immunotherapy. J Cell Physiol. 2019. V. 234. No. 6. P. 8541-8549. DOI: 10.1002/jcp.27816.
- Salmaninejad A., Valilou S. F., Shabgah A. G., et al. PD‐1/PD‐L1 pathway: Basic biology and role in cancer immunotherapy. J Cell Physiol. 2019. V. 234. No. 10. P. 16824-16837. DOI: 10.1002/jcp.28358.
- Sepich-Poore G. D., Zitvogel L., Straussman R., et al. The microbiome and human cancer. Science. 2021. V. 371. No. 6536. Article ID eabc4552. DOI: 10.1126/science.abc4552.
- Seymour L., Bogaerts J., Perrone A., et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017. V. 18. No. 3. P. e143-e152. DOI: 10.1016/S1470-2045(17)30074-8.
- Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018. V. 18. No 3. P. 153-167. DOI: 10.1038/nri.2017.108.
- Sheppard K.A., Fitza L.J., Lee J.M., et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004. V. 574. No. 1-3. P. 37-41. DOI: 10.1016/j.febslet.2004.07.083.
- Singh S., Hassan D., Aldawsari H.M., et al. Immune checkpoint inhibitors: a promising anticancer therapy. Drug Discov Today. 2020. V. 25. No. 1. P. 223-229. DOI: 10.1016/j.drudis.2019.11.003.
- Sivan A., Corrales L., Hubert N., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015. V. 350. No. 6264. P. 1084-1089. DOI: 10.1126/science.aac4255.
- Thorburn A.N., Macia L., Mackay C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014. V. 40. No. 6. P. 833-842. DOI: 10.1016/j.immuni.2014.05.014.
- Valk E., Leung R., Kang H., et al. T cell receptor-interacting molecule acts as a chaperone to modulate surface expression of the CTLA-4 coreceptor. Immunity. 2006. V. 25. No. 5. P. 807-821. DOI: 10.1016/j.immuni.2006.08.024.
- Vestby L.K., Grønseth T., Simm R., et al. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics (Basel). 2020. V. 9. No. 2. Article ID 59. DOI: 10.3390/antibiotics9020059.
- Vétizou M., Pitt J. M., Daillère R., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015. V. 350. No. 6264. P. 1079-1084. DOI: 10.1126/science.aad1329.
- Vivarelli S., Salemi R., Candido S., et al. Gut microbiota and cancer: from pathogenesis to therapy. Cancers. 2019. V. 11. No. 1. Article ID 38. DOI: 10.3390/cancers11010038.
- Wang D.Y., Salem J.E., Cohen J.V., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018. V. 4. No. 12. P. 1721-1728. DOI: 10.1001/jamaoncol.2018.3923.
- Wang Y., Wiesnoski D.H., Helmink B.A., et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018. V. 24. No. 12. P. 1804-1808. DOI: 10.1038/s41591-018-0238-9.
- Weber J., Thompson J. A., Hamid O., et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009. V. 15. No 17. P. 5591-5598. DOI: 10.1158/1078-0432.CCR-09-1024.
- Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020. V. 17. No. 8. P. 807-821. DOI: 10.1038/s41423-020-0488-6.
- Zheng Y., Wang T., Tu X., et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019. V. 7. No 1. P. 1-7. DOI: 10.1186/s40425-019-0650-9.


