Вспомогательные репродуктивные технологии: история становления и роль в развитии генетических технологий в скотоводстве (обзор)
Автор: Зиновьева Н.А., Позябин С.В., Чинаров Р.Ю.
Журнал: Сельскохозяйственная биология @agrobiology
Рубрика: Современные достижения и проблемы генетики и биотехнологии в животноводстве
Статья в выпуске: 2 т.55, 2020 года.
Бесплатный доступ
Разработка технологий активного трансгенеза сделала возможным внесение направленных изменений (геномное редактирование, genome editing, GE) в геном сельскохозяйственных животных с относительно высокой результативностью (обзоры S.Y. Yum с соавт., 2018; A.L. Van Eenennaam, 2019; N.A. Zinovieva с соавт., 2019). Однако эффективное совершенствование систем производства продукции животноводства на основе GE-технологий требует разработки комплексного подхода, основанного на использовании методов биотехнологии, популяционной генетики, геномики количественных признаков и вспомогательных репродуктивных технологий (assisted reproductive technology, ART) (C. Rexroad с соавт., 2019). Развитие ART, включая получение генеративного материала для геномного редактирования от животных с желаемыми генетическими характеристиками, эффективное получение GE-потомства и как можно более раннее его тиражирование, - неотъемлемая составляющая успешного развития и внедрения геномных технологий в скотоводстве (A.L. Van Eenennaam, 2019). В настоящем обзоре проведен ретроспективный анализ развития вспомогательных репродуктивных технологий, в том числе искусственного осеменения (R.H. Foote, 2002; R.G. Saacke, 2012; P. Lonergan, 2018), трансплантации эмбрионов (K.J. Betteridge, 2003; R.J. Mapletoft, 2013), производства эмбрионов in vitro (IVP, in vitro production) (L. Ferré с соавт., 2019), прижизненного получения ооцитов (Ovum-Pick-Up) (R. Boni, 2012; M. Qi с соавт., 2013), переноса ядер соматических клеток (C.L. Keefer, 2015; K.R. Bondioli, 2018; A.V. Lopukhov с соавт., 2019). Дана характеристика современного состояния исследований, дискутируются направления совершенствования ART в связи с применением генетических технологий в скотоводстве, включая генное редактирование. Показано, что за более чем 100-летнюю историю достигнут значительный прогресс в развитии вспомогательных репродуктивных технологий у крупного рогатого скота, многие из которых сегодня активно используются в практическом животноводстве (C. Smith, 1988; L. Ferré с соавт., 2019) и стали базисом для разработки эффективных программ генетического совершенствования скота, включая геномную селекцию (P.M. VanRaden с соавт., 2009). Современные приоритеты в исследованиях ориентированы на прогресс в селекции крупного рогатого скота посредством интеграции GE-технологий в программы разведения (C. Rexroad с соавт., 2019; A.L. Van Eenennaam, 2019). Вспомогательные репродуктивные технологии будут играть одну из определяющих ролей в решении этой амбициозной задачи.
Крупный рогатый скот, вспомогательные репродуктивные технологии, генетические технологии, генное редактирование
Короткий адрес: https://sciup.org/142226291
IDR: 142226291 | УДК: 636.018:591.16 | DOI: 10.15389/agrobiology.2020.2.225rus
Текст обзорной статьи Вспомогательные репродуктивные технологии: история становления и роль в развитии генетических технологий в скотоводстве (обзор)
Развитие ориентированных на сельское хозяйство генетических технологий, включая геномное редактирование, признается мировыми экспертами актуальной задачей современных фундаментальных и прикладных исследований (1, 2). Разработка методов геномной селекции относится к важнейшим научным достижениям последнего десятилетия, применяемым в практике животноводства (1, 3). Один из наиболее ожидаемых научных прорывов следующего десятилетия связывают с технологическим обеспечением рутинного редактирования генов сельскохозяйственных организмов (4). Разработка технологий активного трансгенеза (в последнее время главным образом с использованием CRISPR/Cas9) сделала возможным вне-
Аналитический обзор подготовлен в рамках выполнения работ по теме 0445-2019-0030, финансируемой Минобрнауки России. Результаты экспериментальных исследований влияния гормональной стимуляции и временного режима на результативность OPU получены в рамках проекта РНФ ¹ 19-16-00115.
сение направленных изменений (геномное редактирование, genome editing — GE) в геном сельскохозяйственных животных разных видов с относительно высокой результативностью (5-7). Однако эффективное совершенствование систем производства продукции животноводства на основе GE-технологий требует разработки комплексного подхода, основанного на использовании методов биотехнологии, популяционной генетики, геномики количественных признаков и вспомогательных репродуктивных технологий (1). Развитие вспомогательных репродуктивных технологий, включая получение генеративного материала для геномного редактирования от животных с желаемыми генетическими характеристиками (например, обладающих высокой племенной ценностью по хозяйственно полезным признакам), эффективное получение GE-потомства и как можно более раннее его тиражирование, представляет собой неотъемлемую составляющую успешного развития и имплементации GE-технологий в скотоводстве (6).
В настоящем обзоре проведен ретроспективный анализ развития вспомогательных репродуктивных технологий (ART, assisted reproductive technologies), дана характеристика современного состояния исследований и дискутируются направления совершенствования ART в скотоводстве на основе генетических технологий, включая геномное редактирование.
На ранних этапах основной целью разработки ART было повышение фертильности самцов. Первой такой технологией, использованной на домашних животных, стало искусственное осеменение (artificial insemination — AI). История развития AI у крупного рогатого скота в деталях описана в ряде обзоров (8-10). В настоящей работе мы лишь кратко остановимся на ее основных этапах.
Развитие AI-технологии у млекопитающих началось с 1780 года, когда итальянский физиолог, профессор естествознания Лаззаро Спалланцани (Lazzaro Spallanzani, 1729-1799) впервые провел искусственное осеменение собаки, получив потомство (11, цит. по 12). В 1799 году Джон Хантер (John Hunter, 1728-1793) успешно применил метод Л. Спалланцани на человеке, что привело к рождению здорового ребенка (цит. по 12, 13). Первое использование AI на млекопитающих для увеличения количества потомков, получаемых от одного самца, датировано концом XIX века. В 1885 году заводчик собак породы бассет сэр Эверетт Милле (Everett Millais, 1856-1897) разделил эякулят на три части и использовал этот биоматериал для осеменения трех самок, каждая из которых дала здоровое потомство (цит. по 12, 14). Примерно в это же время эффективность использования AI для повышения фертильности лошадей была продемонстрирована во Франции (15, цит. по 16). Дальнейшее развитие AI-технология получила в работах биолога из Кембриджа Уолтера Хипа (Walter Heape, 18551929), проводившего исследования на собаках, кроликах и лошадях (12). Практические основы искусственного осеменения домашних животных были заложены в 1899 году российским ученым Ильей Ивановичем Ивановым, который предложил использовать этот метод для тиражирования потомства лучших самцов-производителей с целью ускоренного улучшения породных качеств и продуктивности животных (17, 18). Идеи И.И. Иванова получили развитие в работах В.К. Милованова, внедрившего масштабные проекты по искусственному осеменению в скотоводстве и сконструировавшего первые искусственные вагины, подобные которым используются в настоящее время (19). В 1938 году в СССР искусственное осеменение проводилось на 1,2 млн коров.
Повышению роли AI в улучшении генетического потенциала домашних животных способствовало доказательство возможности сохранять оплодотворяющую способность спермиев высших позвоночных при замораживании (криоконсервации), хранении в условиях низких температур и и оттаивании. Впервые это было продемонстрировано в 1942 году на птице: после осеменения кур спермой, которая хранилась при - 79 ° С в течение 1 ч, получили оплодотворенные яйца, однако все зародыши погибли в течение 10-15 ч после оплодотворения (20). Определяющая роль в разработке метода криоконсервации семени домашних животных как основы для широкого практического применения AI-технологии принадлежит советским ученым В.К. Милованову, И.И. Соколовской и И.В. Смирнову. И.И. Соколовской впервые в мире была доказана способность сперматозоидов млекопитающих после замораживания/оттаивания давать начало жизнеспособному потомству: при использовании семени, которое подвергли заморозке в парах углекислого газа и последующему оттаиванию, от крольчих получили 69 нормальных крольчат (21). Это стало научным прорывом, создавшим предпосылки для исследований на других видах сельскохозяйственных животных. Следующая крупная веха — использование глицерина в качестве криопротектора в процессе замораживания и сохранения семени при низких температурах. Хотя в большинстве работ открытие криопротекторной роли глицерина приписывают Christopher Polge (22), еще в 1937 году советские ученые А.Д. Бернштейн и В.В. Петропавловский использовали глицерин для заморозки семени быков, баранов, жеребцов и кроликов при температуре - 21 ° С (23). Однако их работа была опубликована на русском языке и не получила широкого признания. От осеменения заморожено-оттаянным семенем с применением глицерина как криопротектора в 1951 году было получено жизнеспособное потомство у кур (24) и крупного рогатого скота (25), а в 1957 году — у свиней (26) и лошадей (27). Широкое практическое применение в скотоводстве AI-технология получила с конца 1950-х—начала 1960-х годов, составив основу для развития крупномасштабной селекции (28, 29).
Дополнительное преимущество технологии искусственного осеменения дала разработка способа для сортировки сперматозоидов, несущих Х- и Y-хромосомы (30, 31, 32), с помощью измерения содержания ДНК в сперматозоидах млекопитающих методом поточной цитометрии (33). В последующем метод был усовершенствован и нашел широкое практическое применение (31). В настоящее время в странах с развитым скотоводством искусственным осеменением охвачено до 100 % поголовья молочного крупного рогатого скота. Генетический потенциал лучших быков-производителей тиражируется в их потомстве (от нескольких сотен тысяч до более чем миллиона особей) , цит. по 34), что существенно ускоряет генетический прогресс в селекции.
Основные этапы развития методов AI иллюстрирует рисунок 1. AI-технология стала фундаментом для разработки других вспомогательных репродуктивных технологий — пересадки эмбрионов, получения эмбрионов in vitro, клонирования, трансгенеза и генного редактирования.
Не менее актуально мультитиражирование генетического потенциала высокопродуктивных коров. Относительно позднее половое созревание (в возрасте 12-13 мес и старше), малоплодная стельность (как правило, один теленок) и относительно длительный период стельности при использовании традиционной AI-технологии обусловливает получение пер- вого потомства у коров только в возрасте от 2 лет и в дальнейшем рождения (в оптимальных условиях) в среднем одного теленка в год. Проблема мультитиражирования также очень актуальна для реализации программ сохранения редких (малочисленных и генофондных) пород и уникальных генетических ресурсов (например, генетически модифицированных животных). В этой связи задачей развития ART стало как можно более раннее получение большего количества потомства от одной самки.
-
L. Spallanzani: первое успешное AI у млекопитающих (собак)
-
Е. Millais: получение потомства после AI нескольких (трех) самок (собак), осемененных одним эякулятом
И.И. Иванов: практическое развитие AI метода для тиражирования потомства лучших самцов домашних животных
Л.Д. Бернштейн. В, В. Петропавловский: первое использование глицерина в качестве криопротектора для сперматозоидов
В,К. Милованов: масштабирование AI-технологий в скотоводстве, конструирование искусственных вагин
C.S. ShafFner: доказательство способности семени петухов сохранять оплодотворяющую способность после заморозки и оттаивания
И. И. Соколовская: первое получение потомства млекопитающих (кроликов) после AI заморожсно-оттаянным семенем
-
С. Polge: доказательство и широкое мировое признание криопротекторной роли глицерина
D.L. Stewart: получение теленка после AI заморожено-оттаянным семенем
-
D. Pinkel: измерение содержания ДНК в сперматозоидах млекопитающих методом проточной цитометрии
D.L. Garner: разделение X- и Y-содержащих сперматозоидов млекопитающих методом поточной цитометрии
Рис. 1. Основные этапы развития технологии искусственного осеменения (artificial insemination, AI). L. Spallanzani (11), W. Heape (12), E. Millais (14), И.И. Иванов (17), А.Д. Бернштейн, В.В. Петропавловский (23), В.К. Милованов (19), C.S. Shaffner (20), И.И. Соколовская (21), C. Polge с соавт. (22), D. Stewart с соавт. (цит. по 25), D. Pinkel с соавт. (33), D.L. Garner с соавт. (30).
Начало этого направления исследований связывают с разработкой метода трансплантации эмбрионов (ET, embryo transfer) (35, 36). Первый ET-теленок после хирургической пересадки 5-суточного эмбриона, извлеченного из яйцевода коровы после убоя, родился в 1951 году в США (37). На начальных этапах извлечение и пересадку эмбрионов проводили хирургическим путем, что ограничивало широкое практическое применение ET. В 1976 году впервые провели нехирургическое извлечение эмбрионов (38), а в начале 1980-х годов — нехирургическую пересадку эмбрионов у коров (39), что открыло возможность проведения этих работ непосредственно на ферме. Основная цель ранних ET-программ заключалась в распространении в стадах желательных фенотипов. В 1988 году ученые из университета Гвельфа (University of Guelph, Канада) предложили концепцию multiple ovulation embryo transfer (MOET, множественная овуляция и перенос эмбрионов) (40) как способа повышения генетического потенциала стад. Суть MOET заключается в том, что у коров-доноров посредством гормональной обработки вызывают суперовуляцию, затем проводят их искусственное осеменение, на 6-7-е сут после AI эмбрионы вымывают и пересаживают коровам-реципиентам (35, 36). Было показано, что создание нуклеусных стад и так называемый ювенильный MOET в потомстве телок позволяет почти в 2 раза ускорить генетический прогресс по сравнению с традиционными схемами селекции на основе оценки по качеству потом- ства. По данным Международного общества пересадки эмбрионов (International Embryo Transfer Society, IETS, (цит. по. 41), с 1997 до 2005 год наблюдался поступательный рост годового числа MOET-эмбрионов примерно с 450 тыс. до почти 800 тыс., после чего с 2005 по 2013 год производство эмбрионов стабилизировалось в пределах 700-800 тыс/год. В 2014-2016 годах оно снизилось примерно до 610660 тыс/год, главным образом вследствие увеличения количества эмбрионов, получаемых in vitro (цит. по. 41).
Основным недостатком ET-технологии считается необходимость гормональной обработки. Во-первых, известно, что не все доноры одинаково хорошо реагируют на гормональную стимуляцию. Во-вторых, результативность суперовуляции с каждой последующей гормональной обработкой снижается (как правило, результативная реакция на гормональную стимуляцию у коров наблюдается в течение 2-4 последовательных обработок). В-третьих, между гормональными обработками необходим перерыв в 2-3 мес, что увеличивает расходы на содержание коров-доноров. Кроме того, получение эмбрионов невозможно при патологиях яйцеводов (42, 43).
Следующим научным прорывом в развитии ART стало производство эмбрионов in vitro (IVP, in vitro production) (41). Классическая IVP-технология включает выделение ооцитов из яичников самок, созревание полученных ооцитов in vitro (IVM, in vitro maturation), их оплодотворение in vitro (IVF, in vitro fertilization) и развитие in vitro (IVD, in vitro development) до стадий, пригодных к трансплантации или заморозке (как правило, стадии поздней морулы и бластоцисты). Первые телята после оплодотворения in vitro овулировавшей яйцеклетки, созревшей in vivo, родились в 1981 году (44). О телятах, полученных исключительно посредством IVP, включая IVM, IVF и IVD, впервые сообщили в конце 1980-х годов (45). Первоначально для производства IVP-эмбрионов использовали яйцеклетки, извлекаемые из яичников коров после убоя (post mortem), что ограничивало использование этой технологии для генетического улучшения крупного рогатого скота.
Интеграция технологии производства IVP-эмбрионов в программы генетического совершенствования скота началась с разработкой метода прижизненного получения яйцеклеток, известного как Ovum-Pick-Up (OPU) (46, 47). OPU — это неинвазивная процедура извлечения ооцитов из антральных фолликулов у живых животных (48-50). Ооциты коров in vivo впервые получили канадские ученые с помощью метода эндоскопии с доступом через правую паралюмбальную впадину (51). В 1987 году в Дании была предложена аспирация фолликулов коров trans cutaneous под ультразвуковым контролем (52). Следующим шагом стала разработка в 1988 году голландскими учеными метода извлечения ооцитов коров посредством УЗИ-ассистированной трансвагинальной аспирации фолликулов (53). Этот метод вытеснил другие вышеназванные методы и в настоящее время считается базовым для получения ооцитов коров in vivo. В отличие от MOET, OPU не препятствует нормальному воспроизводству и производственному циклу донора (не было выявлено каких-либо долгосрочных негативных последствий для фертильности коров-доноров даже после 2-кратного проведения OPU в неделю в течение более чем года) (54, 55). Подходящим донором может стать любая самка в возрасте от 6 мес до 3-го мес стельности и вскоре после отела (через 2-3 нед) (47). В настоящее время OPU рассматривается как альтернатива традиционной технологии MOET (48,
-
49) и все чаще используется в коммерческих программах во всем мире (50, 56). Совместное использование методов OPU/IVP способно обеспечить получение более 50 телят на одну донорскую корову в год, хотя между донорами наблюдаются существенные различия. Так, T.A.M. Kruip с соавт. (57) проводили OPU дважды в неделю в течение 5 мес и получили в среднем от одной коровы 340 ооцитов и 54 пригодных к трансплантации эмбриона. В 2016 году количество произведенных в мире IVP-эмбрионов коров составило более 600 тыс. и впервые превысило производство MOET-эмбрионов (IETS, цит. по. 41). Учитывая важную роль OPU для развития наиболее передовых генетических технологий, таких как эмбриональная селекция (58) и геномное редактирование (7), остановимся на исследованиях по совершенствованию OPU-технологии более подробно.
Для повышения экономической эффективности OPU-технологии были изучены факторы, влияющие на количество и качество получаемых ооцитов. Оригинальная OPU-технология не предусматривает гормональной стимуляции, что ограничивает количество получаемых яйцеклеток. В этой связи для получения большего количества яйцеклеток за сеанс применяются различные схемы гормональной стимуляции доноров с использованием гонадотропинов плацентарного (например, гонадотропин сыворотки жеребой кобылы, ГСЖК) и гипофизарного (например, фолликулостимулирующий гормон, ФСГ) происхождения (48, 59, 60). Мы изучили влияние гормональной стимуляции с использованием ФСГ на результативность OPU у телок симментальской породы. Обработка ФСГ способствовала увеличению в 3,2 раза (в среднем с 4,5 до 14,6) числа видимых на УЗИ фолликулов (диаметром 3 мм и более), а также числа ооцит-куму-люсных комплексов (ОКК), извлеченных за один сеанс (в среднем с 2,4 до 7,7). При этом различий в качестве ОКК, полученных при гормональной стимуляции и без нее, мы не выявили (61).
Однако при использовании гормональной стимуляции для OPU необходимо учитывать ряд возникающих проблем. Так, экзогенные гормоны нарушают эндокринную систему донора, особенно при длительном применении, что может привести к бесплодию. Реакции разных доноров на гормональную стимуляцию неодинаковы: при применении ФСГ число ооцитов, полученных за сеанс, варьировало от 0 до 26 (62). Даже один и тот же донор в разных сеансах может проявлять неодинаковые реакции, что приводит к нестабильным результатам. В этой связи оптимально использовать гормоны в течение короткого периода, оставляя время для регуляции и восстановления эндокринной системы (47).
На результативность OPU влияет кратность проведения сессий OPU, их технические и технологические параметры, индивидуальные особенности доноров (порода, возраст, репродуктивная фаза, реакция организма), обеспеченность рациона необходимыми питательными веществами (63), климатические условия (64, 65) и опыт оператора (47).
Классическая процедура OPU (без гормональной стимуляции) в большинстве случаев предусматривает 2-кратное проведение пункции за неделю (2/w). Выбор в пользу режима 2/w обусловлен тем, что в этом случае увеличивается частота фолликулярных волн, происходит задержка эстрального цикла, созревания фолликулов и овуляции. Животные, подвергшиеся OPU в режиме 2/w, вступают в так называемое парафизиологиче-ское состояние, при котором фолликулярные волны не зависят от эстрального цикла (57). При использовании режима 2/w не развивается до-230
минантный фолликул, так как все видимые фолликулы аспирируются в процессе OPU. При проведении OPU в режиме раз в неделю (1/w) и реже в большинстве случаев развивается доминантный фолликул, что приводит к регрессии и вырождению субординатных фолликулов.
Сравнительный анализ OPU в режиме 1/w и 2/w не выявил различий в количестве аспирированных фолликулов, извлеченных ооцитов и бластоцист, полученных на 7-и сут культивирования, в расчете на одну корову за сеанс. Однако в расчете на неделю все эти три показателя были значительно выше для режима 2/w по сравнению с 1/w (66-68). Нами было изучено влияние двух различных временных режимов на результативность OPU у телок симментальской породы в отношении количества и качества получаемых ооцитов (69). В среднем число ооцитов от донора за сессию при использовании обоих режимов оставило 4,4. Мы установили достоверное повышение в 1,2 раза (p < 0,05) доли OPU-ооцитов хорошего качества, характеризующихся нормальной морфологией , при выполнении процедуры в режиме 2/w (65,7±4,0 % от общего числа извлеченных ооцитов) по сравнению с 1/w (53,6±3,0 %). Степень созревания ооцитов (74,0 %), степень дробления оплодотворенных ооцитов (в среднем 63,5 %) и степень развития эмбрионов до стадии бластоцисты (в среднем 16,7 %) при этом были сопоставимы. В результате выполнение процедуры OPU дважды в неделю (с учетом повышения доли ооцитов хорошего качества по сравнению с проведением OPU-сессий один раз в неделю) позволяло за определенный период времени получать от одного донора в 2,5 раза больше эмбрионов в стадии бластоцисты (69).
Установлено влияние возраста, а также физиологического состояния на результативность OPU. D. Rizos с соавт. (70) показали более высокую результативность OPU у телок голштинской породы по сравнению с коровами: общее число извлеченных ооцитов составило соответственно 4,7 против 2,8 (для ооцитов 1-2-й степени — 3,0 против 1,8). Существенных различий в степени дробления оплодотворенных яйцеклеток и выходе бластоцист между телками и коровами не наблюдалось (70).
Установлено влияние физиологического состояния на способность OPU-ооцитов к дальнейшему развитию. В эксперименте на черном японском скоте было показано, что процент дробления оплодотворенных яйцеклеток и их развития до стадии бластоцисты, а также выживаемость после заморозки выше для эмбрионов, полученных из ооцитов, извлеченных у стельных коров по сравнению с нестельными коровами (71).
Выявлены существенные различия в результативности OPU между породами Bos taurus и Bos indicus (зебувидный скот). От доноров зебувид-ных пород получают существенно больше ооцитов (72, 73), главным образом вследствие большего размера популяции фолликулов в яичнике. От доноров зебувидного скота породы Нелоре, разводимой в Бразилии, было получено от 18 до 25 ооцитов без использования экзогенных гормонов или протоколов синхронизации (74, 75). Сравнительное исследование голштинской породы (Bos taurus) и породы гир (Bos indicus) выполнили J.H.F. Pontes с соавт. (50). Число жизнеспособных ооцитов, извлеченных за один сеанс OPU, у коров-доноров голштинской породы, породы гир, кроссов 1/2 голштинская ½ 1/2 гир и 1/4 голштинская ½ 3/4 гир составило соответственно 8,0±2,7; 12,1±3,9; 24,3±4,7 и 16,8±5,0. Доля IVP-эмбрионов, полученных от осеменения сексированным семенем (36-40 %), существенно не различалась между группами (50). Между разными породами скота Bos taurus заметных различий в результативности OPU не выявлено. Для прогнозирования количества антральных фолликулов в яичниках коров пород Bos taurus и Bos indicus и, как следствие, результативности OPU можно использовать концентрацию антимюллерова гормона в плазме крови (он вырабатывается клетками фолликулов в период их созревания) (76).
Технические факторы, влияющие на результативность OPU, — это чувствительность приборов для УЗИ-диагностики, тип (секторный или линейный) и частота используемого зонда (57, 77), характеристики вакуума (78-80), диаметр и длина скоса игл (57, 81, 82), прокручивание иглы внутри фолликула в процессе аспирации (80, 83), удаление доминантного фолликула (66, 84).
Еще одну возможность получения ооцитов от живых коров дает лапароскопический метод сбора ооцитов (L-OPU), который на крупном рогатом скоте впервые применили в 1992 году (85). L-OPU обладает преимуществами по сравнению с классической процедурой OPU: можно выбирать для аспирации поверхностные фолликулы, аспирировать фолликулы меньшего диаметра (от 2 мм), вести непосредственное наблюдение за репродуктивными органами и яичником, осуществлять визуальный контроль процедуры аспирации, что снижает риск повреждения яичника. В то же время сравнительные исследования показали, что при использовании классической технологии OPU число ооцитов хорошего качества и, как следствие, выход эмбрионов на стадии морулы/бластоцисты выше, чем при L-OPU (86, 87). Технология L-OPU находит применение для получения ооцитов от препубертальных самок (в возрасте от 2 мес), на которых использование классической OPU-технологии невозможно. Методом L-OPU от телочек в возрасте 2-6 мес за одну сессию было получено 4,6 (85), 21,4 (88) и 42,6 ооцита (89). Отбор (на основе геномной оценки) препуберталь-ных самок с высокой племенной ценностью в качестве доноров ооцитов позволяет уменьшить генерационный интервал и, как следствие, ускорить генетически прогресс в селекции (90). Однако для практического применения этой технологии необходимо совершенствование протоколов получения эмбрионов in vitro с использованием ювенальных ооцитов.
H.D. Reichenbach с соавт. (91) предложили модификацию метода L-OPU с доступом к яичникам коров через свод влагалища. Процедура проводится под эпидуральной анестезией менее чем за 15 мин, не требует хирургического вмешательства и может выполняться в полевых условиях.
Еще одним научным прорывом в развитии ART как фундамента для развития передовых генетических технологий в скотоводстве стало успешное эмбриональное клонирование с использованием соматических клеток — SCNT (somatic cell nuclear transfer). SCNT — это метод, при котором ядро соматической клетки переносится в энуклеированный ооцит для получения нового индивидуума, генетически идентичного донору соматической клетки (92-94). Рождение первых клонированных телят было описано в 1993 году (95). Для клонирования использовали клетки внутренней клеточной массы (ICM, inner cell mass), которые выделяли из бластоцист и культивировали от 6 до 100 сут. О первых телятах, полученных методом SCNT с использованием дифференцированных соматических клеток (фетальных фибробластов), сообщили в 1998 году (96).
Таким образом, за столетний период в скотоводстве были разработаны и внедрены в практику различные ART (рис. 2), которые стали основой для эффективных технологий генетического улучшения крупного ро-232
гатого скота, включая технологии геномной селекции (3, 97).
И.И. Иванов: получение потомства от искусственного осеменения коров
-
D, Stewart: рождение теленка после осеменения заморожепо-оттаянным семенем
E.L. Willett: рождение первого ЕТ-теленка после хирургической пересадки 5-суточного эмбриона
R.P. Elsden; нехирургическое извлечение эмбрионов коров
R.F. Rowe: нехирургическая пересадка эмбрионов у коров
B.G. Brackett: рождение теленка после оплодотворения in vitro овулировавшей яйцеклетки, созревшей in vivo
К. Goto: рождение теленка, полученного исключительно из ТУР-эмбриона, включая ГУМ, ГУТ и ГУР
-
С. Smith: МОЕТ для улучшения генетического потенциала стад
М.С. Pieterse: прижизненное получение ооцитов коров посредством УЗИ-ассистированной трансвагинальной аспирации фолликулов (Ovum-Pick-Up)
М. Sims, N.L First: рождение первых телят, полученных методом SCNT, с использованием ICM-клеток. культивированных in vitro, в качестве доноров
-
X. Vignon: рождение первых телят, полученных методом SCNT, с использованием дифференцированных соматических клеток в качестве доноров
Рис. 2. Этапы разработки вспомогательных репродуктивных технологий (assisted reproductive technology, ART), ставшие основой для развития генетических технологий в скотоводстве. И.И. Иванов (17), D. Stewart с соавт. (цит. по 25), E.L. Willett с соавт. (37), R.P. Elsden с соавт. (38), R.F. Rowe с соавт. (39), B.G. Brackett с соавт. (44), K. Goto с соавт. (45), C. Smith с соавт. (40), M.C. Pieterse с соавт. (53), M. Sims, N.L. First (95), X. Vignon с соавт. (96); ET — пересадка эмбрионов, IVP — получение эмбрионов in vitro, IVM — созревание in vitro, IVF — оплодотворение in vitro, IVD — развитие in vitro, MOET — множественная овуляция и пересадка эмбрионов, SCNT — перенос ядер соматических клеток, ICM — внутренняя клеточная масса.
Совершенствование ART, включая технологии IVP и ET, до уровня рутинной лабораторной практики инициировало попытки внесения генетических изменений в ранние эмбрионы сельскохозяйственных животных. На начальном этапе развития методов трансгенеза для этих целей использовались микроинъекции раствора генных конструкций в пронуклеус зигот (5, 98, 99). Результативность такого подхода для генерации трансгенных млекопитающих была первоначально продемонстрирована на мышах (100). Впервые о создании трансгенных сельскохозяйственных животных сообщили в 1985 году две лаборатории — в США (101) и Германии (102). Первые трансгенные телята, несущие ген лактоферрина человека под контролем промотора гена α-S1-казеина крупного рогатого скота, были получены в 1991 году (103). В последующие годы с использованием метода микроинъекции выполнялись различные генетические модификации у сельскохозяйственных животных разных видов, включая крупный рогатый скот (104). Основные недостатки метода микроинъекции — его очень высокая трудоемкость и низкая эффективность. Для получения одного трансгенного теленка необходимо инъецировать более 1000 зигот (105). При этом только около 70 % трансгенных животных-родоначальников способны передавать трансген по наследству потомству, а из полученных трансгенных линий только у 50 % уровень экспрессии достаточен для последующего практического использования (106). Из-за высокой стоимости метода микроинъекций основные цели генетической модификации домашних животных переместились из сельскохозяйственной области в биомедицинскую, где возможны более высокие доходы от внедрения (107). В середине 1990-х годов метод пересадки ядер соматических клеток, генетически трансформированных in vitro, практически полностью вытеснил микроинъекции в пронуклеус зигот (рис. 3).
Извлечение Созревание Энуклеация ооцитов ооцитов ооцитов

Животное -донор клеток
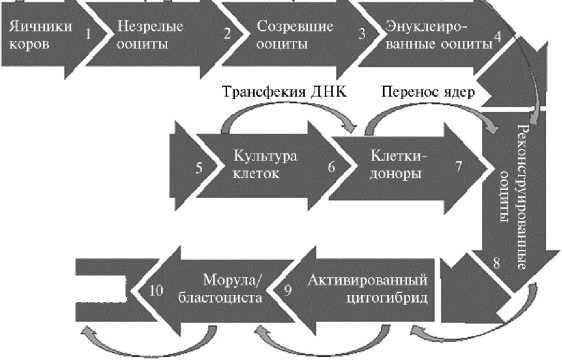
Культура
Клетки доноры
Яичники коров
Незрелые ооциты
Морула/ бластоциста
.Энуклеиро ванные ооциты'
Созревшие ооциты
Траисфекия ДНК
Перенос ядер
Активированный цитогибрид
Пересадка реципиентам
Электрослияние и активация
Культивирование in vitro
Рис. 3. Схема создания генетически модифицированных животных с использованием технологии переноса ядер соматических клеток (somatic cell nuclear transfer, SCNT).
Трансгенное потомство
Преимущества SCNT по сравнению с методом микроинъекции заключаются в том, что SCNT позволяет выбирать клетки-доноры определенного пола и проводить предварительный отбор клеток с заданными генетическими изменениями в культуре in vitro, в результате чего 100 % полученных потомков будут иметь желаемый пол и нести нужные генетические модификации. Еще одно преимущество SCNT в аспекте последующего применения для сельскохозяйственных целей — это возможность получения доноров ядер от высокопродуктивных животных, а также высокодостоверное прогнозирование племенной ценности будущего потомства с помощью геномной оценки (6). К недостаткам метода следует отнести пониженную жизнеспособность эмбрионов, полученных методом SCNT, что проявляется в большей (на 60 %) эмбриональной смертности между 35-ми и 60-ми сут стельности по сравнению с IVP-эмбрионами (108). После сообщения в 1998 году о первом трансгенном теленке, несущем репортерные гены β -галаксидазы и неомицина (109), метод SCNT более 15 лет оставался доминирующим при получении трансгенного крупного рогатого скота.
За более чем 30-летний период разработано большое число приемов трансгенеза, которые в сочетании с различными ART были успешно применены для генерации генетических изменений у крупного рогатого скота (рис. 4). Однако использование технологий трансгенеза в программах селекционно-племенной работы в скотоводстве до недавнего времени лимитировалось относительно высокой стоимостью создания трансгенного крупного рогатого скота, а также отсутствием надежного метода, способного с высокой эффективностью обеспечить внесение заданных генетических изменений в целевые участки генома (5, 6).
Дальнейший прогресс в области генной инженерии домашних жи- вотных связывают с развитием технологий геномного редактирования (genome editing, GE), обеспечивающих возможность генераций направленных (сайт-специфических) модификаций в геноме (120). В качестве инструментов для GE крупного рогатого скота находят применение системы ДНК-транспозонов (118) и сайт-специфических нуклеаз, включая ZFN (zinc-finger nucleases) нуклеазы «цинковых пальцев» (114, 115), TALEN (transcription activator-like effector nucleases) — эффекторные нуклеазы, подобные активаторам транскрипции (116, 117), и системы на основе CRISPR/Cas9 (CRISPR — короткие палиндромные повторы, регулярно расположенные группами; Cas9 — CRISPR-ассоциированный белок 9) (120). Вследствие относительной простоты создания генных конструкций последние приобретают все большую популярность для GE сельскохозяйственных животных (7).

P. Krimpenfort: инъекция ДНК в пронуклеус зигот
J.B. Cibelli: соматическое клонирование (SCNT)
A.W.S. Chan: ретровирусный перенос ДНК
Y. Kuroiwa: трансхромосомалъный перенос ДНК via SCNT
A. Hofmann: ленгивирусный перенос ДНК
J.A. Richt: нокаут посредством гомологичной рекомбинации
S. Yu: нокаут посредством ZNF
-
X. Liu: knock-in посредством ZNF
С. Proudfoot, Н. Wu: нокаут и knock-in посредством TALEN
S.Y. Yum: использование транспозонов
Y. Gao: knock-in посредством CRISPR/Cas9
Рис. 4. Развитие методов генетической модификации крупного рогатого скота. P. Krimpenfort с соавт. (103), J.B. Cibelli с соавт. (109), A.W.S. Chan с соавт. (110), Y. Kuroiwa с соавт. (111), A. Hofmann с соавт. (112), J.A. Richt с соавт. (113), S. Yu с соавт. (114), X. Liu с соавт. (115), C. Proudfoot с соавт. (116), H. Wu с соавт. (117), S.Y. Yum с соавт. (118), Y. Gao с соавт. (119).
Для заданных генетических изменений в генеративных линиях сельскохозяйственных животных посредством GE используются два основных подхода: пересадка ядер соматических клеток (как правило, эмбриональных фибробластов), предварительно модифицированных in vitro (см. рис. 2), и микроинъекция РНК-формы генных конструкций в зиготы. Преимущества SCNT отмечались выше, однако SCNT все еще не стал рутинной процедурой во многих лабораториях (121). Выполнить микроинъекции относительно проще. В отличие от классической микроинъекции в пронуклеус зигот (101, 102), при использовании сайт-специфических нуклеаз генные конструкции вводят в цитоплазму зигот. Несмотря на то, что только часть животных, полученных из инъецированных эмбрионов, несут ожидаемые генетические изменения, метод микроинъекции был успешно реализован при GE у крупного рогатого скота (7). В сочетании с технологией OPU/IVP, позволяющей в большом количестве получать зиготы от родителей с высокой племенной ценностью, метод микроинъекции может стать базовым для использования в программах генетического улучшения крупного рогатого скота посредством GE.
Итак, за более чем 100-летнюю историю достигнут значительный прогресс в развитии вспомогательных репродуктивных технологий для крупного рогатого скота. Такие технологии, как искусственное осеменение (в том числе с использованием семени, разделенного по полу), множественная овуляция и пересадка эмбрионов, в настоящее время активно используются в практическом животноводстве и стали базисом для разработки эффективных программ генетического совершенствования поголовья, включая геномную селекцию. Дальнейший прогресс в селекции крупного рогатого скота связывают с интеграцией в программы разведения технологии геномного редактирования. Успешное решение этой амбициозной задачи во многом будет зависеть от развития вспомогательных репродуктивных технологий, таких как прижизненное получение яйцеклеток коров, оплодотворение и культивирование эмбрионов in vitro, пересадка ядер генетически модифицированных соматических клеток.
Список литературы Вспомогательные репродуктивные технологии: история становления и роль в развитии генетических технологий в скотоводстве (обзор)
- Rexroad C., Vallet J., Matukumalli L.K., Reecy J., Bickhart D., Blackburn H., Boggess M., Cheng H., Clutter A., Cockett N., Ernst C., Fulton J.E., Liu J., Lunney J., Neibergs H., Purcell C., Smith T.P.L., Sonstegard T., Taylor J., Telugu B., Van Eenennaam A., Van Tassell C.P., Wells K. Genome to phenome: improving animal health, production, and well-being - a new USDA blueprint for animal genome research 2018-2027. Frontiers in Genetics, 2019, 10: 327 ( ). DOI: 10.3389/fgene.2019.00327
- Федеральная научно-техническая программа развития генетических технологий на 2019-2027 годы. Утверждена Постановлением Правительства Российской Федерации от 22 апреля 2019 года № 479.
- VanRaden P.M., Van Tassell C.P., Wiggans G.R., Sonstegard T.S., Schnabel R.D., Taylor J.F., Schenkel F.S. Invited review: reliability of genomic predictions for North American Holstein bulls. Journal of Dairy Science, 2009, 92(1): 16-24 ( ). DOI: 10.3168/jds.2008-1514
- Science breakthroughs to advance food and agricultural research by 2030. A consensus study report of the National Academies of Sciences, Engineering and Medicine. The National Academies Press, Washington, DC, 2019 ( ). DOI: 10.17226/25059
- Yum S.Y., Youn K.Y., Choi W.J., Jang G. Development of genome engineering technologies in cattle: from random to specific. Journal of Animal Science and Biotechnology, 2018, 9: 16 ( ). DOI: 10.1186/s40104-018-0232-6
- Van Eenennaam A.L. Application of genome editing in farm animals: cattle. Transgenic Research, 2019, 28: 93-100 ( ).
- DOI: 10.1007/s11248-019-00141-6
- Zinovieva N.A., Volkova N.A., Bagirov V.A. Genome editing: current state of research and application to animal husbandry. Applied Biochemistry and Microbiology, 2019, 55(7): 711-721 ( ).
- DOI: 10.1134/S000368381907007X
- Foote R.H. The history of artificial insemination: selected notes and notables. Journal of Animal Science, 2002, 80(2): 1-10.
- Saacke R.G. AI: a historical perspective. Proc. of the 24th Technical Conference on Artificial insemination and reproduction. Milwaukee, WI, USA, 2012: 138-150.
- Lonergan P. Review: Historical and futuristic developments in bovine semen technology. Animal, 2018, 12(S1): s4-s18 ( ).
- DOI: 10.1017/S175173111800071X
- Spallanzani L. Experiences pour servir a l'liistoire de la generation des animaux et des plantes. Geneve, 1786.
- Heape W. The artificial insemination of mammals and subsequent possible fertilisation or impregnation of their ova. Proceedings of the Royal Society of London, 1897, 61(369-377): 52-63 ( ).
- DOI: 10.1098/rspl.1897.0012
- Home E. An account of the dissection of an hermaphrodite dog. To which are prefixed, some observations on hermaphrodites in general. By Everard Home, Esq. F. R. S. Philosophical Transactions of the Royal Society, 1799, 89: 157-178. Режим доступа: https://www.jstor.org/stable/107031. Дата обращения 21.04.2020.
- Millais E. Influence with special reference to that of sire. In: The Dog Owners' Annual for 1894. London, Dean, 1894: 153.
- Herman H.A. Improving cattle by the millions: NAAB and the development and worldwide application of artificial insemination. University of Missouri Press, Columbia, 1981.
- Walters E.M., Benson J.D., Woods E.J., Critser J.K. The history of sperm cryopreservation importance of sperm cryopreservation. In: Sperm banking: theory and practice /A.A. Pacey, M.J. Tomlinson (eds.). Cambridge University Press, 2009.
- Иванов И.И. Искусственное оплодотворение у млекопитающих. Экспериментальное исследование. Архив биологических наук, 1906, 12(4-5): 376-509.
- Ivanoff E.I. On the use of artificial insemination for zootechnical purposes in Russia. The Journal of Agricultural Science, 1922, 12(3): 244-256 ( ).
- DOI: 10.1017/s002185960000530x
- Милованов В.К. Искусственное осеменение сельскохозяйственных животных. М., 1938.
- Shaffner C.S. Longevity of fowl spermatozoa in frozen condition. Science, 1942, 96(2493): 377 ( ).
- DOI: 10.1126/science.96.2493.337
- Соколовская И.И. Может ли замороженная сперма оплодотворять и давать нормальное потомство. Доклады ВАСХНИЛ, 1947, 6: 21-23.
- Polge C., Smith A.U., Parkes A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature, 1949, 164: 666 ( ).
- DOI: 10.1038/164666a0
- Бернштейн А.Д., Петропавловский В.В. Влияние неэлектролитов на переживание сперматозоидов. Бюллетень экспериментальной биологии и медицины, 1937, 1: 21-25.
- Polge C. Functional survival of fowl spermatozoa after freezing at -79 °С. Nature, 1951, 167: 949-950 ( ).
- DOI: 10.1038/167949b0
- Smith A.U., Polge C. Storage of bull spermatozoa at low temperatures. Veterinary Record, 1950, 62: 115-116.
- Hess E.A., Teague H.S., Ludwick T.M., Martig R.C. Swine can be bred with frozen semen. Ohio Farm Home Res., 1957, 42: 100
- Barker C.A.V., Grandier J.C.C. Pregnancy in a mare resulted from frozen epididymal spermatozoa. Can. J. Comp. Med. Vet. Sci., 1957, 21(2): 47-51.
- Милованов В.К., Соколовская И.И. Крупномасштабная селекция в животноводстве и искусственное осеменение. Вестник сельскохозяйственной науки, 1980, 12: 122-134.
- Эрнст Л.К., Цалитис А.А. Крупномасштабная селекция в скотоводстве. М., 1982.
- Garner D.L., Gledhill B.L., Pinkel D., Lake S., Stephenson D., Van Dilla M.A., Johnson L.A. Quantification of the X- and Y-chromosome-bearing spermatozoa of domestic animals by flow cytometry. Biology of Reproduction, 1983, 28(2): 312-321 ( ).
- DOI: 10.1095/biolreprod28.2.312
- Seidel G.E. Jr. Update on sexed semen technology in cattle. Animal, 2014, 8(s1): 160-164 ( ).
- DOI: 10.1017/S1751731114000202
- Rath D., Barcikowski S., de Graaf S., Garrels W., Grossfeld R., Klein S., Knabe W., Knorr C., Kues W., Meyer H., Michl J., Moench-Tegeder G., Rehbock C., Taylor U., Washausen S. Sex selection of sperm in farm animals: status report and developmental prospects. Reproduction, 2013, 145(1): R15-R30 ( ).
- DOI: 10.1530/REP-12-0151
- Pinkel D., Lake S., Gledhill B.L., Van Dilla M.A., Stephenson D., Watchmaker G. High resolution DNA content measurements of mammalian sperm. Cytometry, 1982, 3(1): 1-9 ( ).
- DOI: 10.1002/cyto.990030103
- Zinovieva N.A. Haplotypes affecting fertility in Holstein cattle. Sel'skokhozyaistvennaya Biologiya [Agricultural Biology], 2016, 51(4): 423-435 ( ).
- DOI: 10.15389/agrobiology.2016.4.423eng
- Betteridge K.J. A history of farm animal embryo transfer and some associated techniques. Animal Reproduction Science, 2003, 79(3-4): 203-244 (
- DOI: 10.1016/S0378-4320(03)00166-0)
- Mapletoft R.J. History and perspectives on bovine embryo transfer. Animal Reproduction, 2013, 10(3): 168-173
- Willett E.L., Black W.G., Casida L.E., Stone W.H., Buckner P.J. Successful transplantation of a fertilized bovine ovum. Science, 1951, 113(2931): 247 ( ).
- DOI: 10.1126/science.113.2931.247
- Elsden R.P., Hasler J.F., Seidel G.E. Jr. Non-surgical recovery of bovine eggs. Theriogenology, 1976, 6(5): 523-532 (
- DOI: 10.1016/0093-691X(76)90120-5)
- Rowe R.F., Del Campo M.R., Critser J.K., Ginther O.J. Embryo transfer in cattle: nonsurgical transfer. Am. J. Vet. Res., 1980, 41(7): 1024-1028.
- Smith C. Applications of embryo transfer in animal breeding. Theriogenology, 1988, 29(1): 203-212 (
- DOI: 10.1016/0093-691X(88)90040-4)
- Ferré L., Kjelland M., Strøbech L., Hyttel P., Mermillod P., Ross P. Recent advances in bovine in vitro embryo production: reproductive biotechnology history and methods. Animal, 2020, 14(5): 991-1004 ( )
- DOI: 10.1017/S1751731119002775
- Эрнст Л.К., Сергеев Н.И. Трансплантация эмбрионов сельскохозяйственных животных. М., 1989.
- Прокофьев М.И. Регуляция воспроизводства крупного рогатого скота. М., 1989.
- Brackett B.G., Bousquet D., Boice M.L., Donawick W.J., Evans J.F., Dressel M.A. Normal development following in vitro fertilization in the cow. Biology of Reproduction, 1982, 27(1): 147-158 ( ).
- DOI: 10.1095/biolreprod27.1.147
- Goto K., Kajihara Y., Kosaka S., Koba M., Nakanishi Y., Ogawa K. Pregnancies after co-culture of cumulus cells with bovine embryos derived from in-vitro fertilization of in-vitro matured follicular oocytes. Journal of Reproduction and Fertility, 1988, 83(2): 753-758 ( ).
- DOI: 10.1530/jrf.0.0830753
- Boni R. Ovum pick-up in cattle: a 25 years retrospective analysis. Animal Reproduction, 2012, 9(3): 362-369.
- Qi M., Yao Y., Ma H., Wang J., Zhao X., Liu L., Tang X., Zhang L., Zhang S., Sun F. Transvaginal ultrasound guided Ovum Pick-up (OPU) in cattle. Journal of Biomimetics Biomaterials and Tissue Engineering, 2013, 18: 118 ( ).
- DOI: 10.4172/1662-100X.1000118
- Kruip T.A.M., Pieterse M.C., van Beneden T.H., Vos P.L., Wurth Y.A., Taverne M.A. A new method for bovine embryo production: a potential alternative to superovulation. Veterinary Record, 1991, 128(9): 208-210 ( ).
- DOI: 10.1136/vr.128.9.208
- Bousquet D., Twagiramungu H., Morin N., Brisson C., Carboneau G., Durocher J. In vitro embryo production in the cow: an effective alternative to the conventional embryo production approach. Theriogenology, 1999, 51(1): 59-70 (
- DOI: 10.1016/S0093-691X(98)00231-3)
- Pontes J.H.F., Silva K.C.F., Basso A.C., Rigo A.G., Ferreira C.R., Santos G.M.G., Sanches B.V., Porcionato J.P., Vieira P.H., Faifer F.S., Sterza F.A., Schenk J.L., Seneda M.M. Large-scale in vitro embryo production and pregnancy rates from Bos taurus, Bos indicus, and indicus-taurus dairy cows using sexed sperm. Theriogenology, 2010, 74(8): 1349-1355 ( 10).
- DOI: 10.1016/j.theriogenology.2010.06.004
- Lambert R.D., Bernard C., Rioux J.E., Béland R., D'Amours D., Montreuil A. Endoscopy in cattle by the paralumbar route: technique for ovarian examination and follicular aspiration. Theriogenology, 1983, 20(2): 149-161 (
- DOI: 10.1016/0093-691X(83)90210-8)
- Callesen H., Greve T., Christensen F. Ultrasonically guided aspiration of bovine follicular oocytes. Theriogenology, 1987, 27(1): 217 (
- DOI: 10.1016/0093-691X(87)90094-X)
- Pieterse M.C., Kappen K.A., Kruip T.A.M., Taverne M.A.M. Aspiration of bovine oocytes during transvaginal ultrasound scanning of the ovaries. Theriogenology, 1988, 30(4): 751-762 (
- DOI: 10.1016/0093-691X(88)90310-X)
- Galli C., Crotti G., Notari C., Turini P., Duchi R., Lazzari G. Embryo production by ovum pick up from live donors. Theriogenology, 2001, 55(6): 1341-1357 (
- DOI: 10.1016/S0093-691X(01)00486-1)
- Chastant-Maillard S., Quinton H., Lauffenburger J., Cordonnier-Lefort N., Richard C., Marchal J., Mormede P., Renard J.P. Consequences of transvaginal follicular puncture on well-being in cows. Reproduction, 2003, 125(4): 555-563 ( ).
- DOI: 10.1530/rep.0.1250555
- Faber D.C., Molina J.A., Ohlrichs C.O., Vander Zwaag D.F., Ferre L.B. Commercialization of animal biotechnology. Theriogenology, 2003, 59(1): 125-138 (
- DOI: 10.1016/S0093-691X(02)01264-5)
- Kruip T.A.M., Boni R., Wurth Y.A., Roelofsen M.W.M., Pieterse M.C. Potential use of ovum pick-up for embryo production and breeding in cattle. Theriogenology, 1994, 42(4): 675-683 (
- DOI: 10.1016/0093-691X(94)90384-U)
- Wu B., Zan L., Quan F., Wang H. A novel discipline in embryology - animal embryo breeding. In: New discoveries in embryology /B. Wu (ed.). IntechOpen Limited, London, 2015: Ch. 8 ( ).
- DOI: 10.5772/61299
- Chaubal S.A., Ferre L.B., Molina J.A., Faber D.C., Bols P.E., Rezamand P., Tian X., Yang X. Hormonal treatments for increasing the oocyte and embryo production in an OPU-IVP system. Theriogenology, 2007, 67(4): 719-728 ( ).
- DOI: 10.1016/j.theriogenology.2006.07.022
- Sendag S., Cetin Y., Alan M., Hadeler K.G., Niemann H. Effects of eCG and FSH on ovarian response, recovery rate and number and quality of oocytes obtained by ovum pick-up in Holstein cows. Animal Reproduction Science, 2008, 106(1-2): 208-214 ( ).
- DOI: 10.1016/j.anireprosci.2008.01.007
- Чинаров Р.Ю., Тарадайник Н.П., Тарадайник Т.Е., Сингина Г.Н., Позябин С.В. Сравнительное исследование количественных и качественных показателей результативности трансвагинального извлечения ооцитов коров при использовании и отсутствии гормональной стимуляции. В сб.: Науч. тр. учебно-методической и научно-практической конференции, посвященной 100-летию со дня основания ФГБОУ ВО МГАВМиБ - МВА им. К.И. Скрябина "Актуальные проблемы ветеринарной медицины, зоотехнии и биотехнологии". М., 2019: 377-378.
- De Roover R., Bols P.E.J., Genicot G., Hanzen Ch. Characterisation of low, medium and high responders following FSH stimulation prior to ultrasound- guided transvaginal oocyte retrieval in cows. Theriogenology, 2005, 63(7): 1902-1913 ( ).
- DOI: 10.1016/j.theriogenology.2004.08.011
- Leroy J.L.M.R., Van Soom G., Opsomer G., Goovaerts I.G.F., Bols P.E.J. Reduced fertility in high-yielding dairy cows: Are the oocyte and embryo in danger? Part II. Mechanisms linking nutrition and reduced oocyte and embryo quality in high-yielding dairy cows. Reproduction in Domestic Animals, 2008, 43(5): 623-632 ( ).
- DOI: 10.1111/j.1439-0531.2007.00961.x
- Ferreira R.M., Ayres H., Chiaratti M.R., Ferraz M.L., Araújo A.B., Rodrigues C.A., Watanabe Y.F., Vireque A.A., Joaquim D.C., Smith L.C., Meirelles F.V., Baruselli P.S. The low fertility of repeat-breeder cows during summer heat stress is related to a low oocyte competence to develop into blastocysts. Journal of Dairy Science, 2011, 94(5): 2383-2392 ( ).
- DOI: 10.3168/jds.2010-3904
- Ferreira R.M., Chiaratti M.R., Macabelli C.H., Rodrigues C.A., Ferraz M.L., Watanabe Y.F., Smith L.C., Meirelles F.V., Baruselli P.S. The infertility of repeat-breeder cows during summer is associated with decreased mitochondrial DNA and increased expression of mitochondrial and apoptotic genes in oocytes. Biology of Reproduction, 2016, 94(66): 1-10 ( ).
- DOI: 10.1095/biolreprod.115.133017
- Chaubal S.A., Molina J.A., Ohlrichs C.L., Ferre L.B., Faber D.C., Bols P.E., Riesen J.W., Tian X., Yang X. Comparison of different transvaginal ovum pick-up protocols to optimise oocyte retrieval and embryo production over a 10-week period in cows. Theriogenology, 2006, 65(8): 1631-1648 ( ).
- DOI: 10.1016/j.theriogenology.2005.07.020
- Lopes A.S., Martinussen T., Greve T., Callesen H. Effect of days post-partum, breed and ovum pick-up scheme on bovine oocyte recovery and embryo development. Reproduction in Domestic Animals, 2006, 41(3): 196-203 ( ).
- DOI: 10.1111/j.1439-0531.2006.00683.x
- Li F., Chen X., Pi W., Liu C., Shi Z. Collection of oocytes through transvaginal ovum pick-up for in vitro embryo production in Nanyang Yellow cattle. Reproduction in Domestic Animals, 2007, 42(6): 666-670 ( ).
- DOI: 10.1111/j.1439-0531.2006.00842.x
- Чинаров Р.Ю., Луканина В.А., Сингина Г.Н., Тарадайник Н.П. Результативность получения ооцитов коров при использовании различных временных режимов трансвагинальной пункции фолликулов. Достижения науки и техники АПК, 2020, 34(2): 57-60 ( ).
- DOI: 10.24411/0235-2451-2020-10212
- Rizos D., Burke L., Duffy P., Wade M., Mee J.F., O'Farrell K.J., Macsiurtain M., Boland M.P., Lonergan P. Comparisons between nulliparous heifers and cows as oocyte donors for embryo production in vitro. Theriogenology, 2005, 63(3): 939-949 ( ).
- DOI: 10.1016/j.theriogenology.2004.05.008
- Takuma T., Sakai S., Ezoe D., Ichimaru H., Jinnouchi T., Kaedei Y., Nagai T., Otoi T. Effects of season and reproductive phase on the quality, quantity and developmental competence of oocytes aspirated Japanese Black cows. Journal of Reproduction and Development, 2010, 56(1): 55-59 ( ).
- DOI: 10.1262/jrd.09-071h
- Guerreiro B.M., Batista E.O., Vieira L.M., Sá Filho M.F., Rodrigues C.A., Castro Netto A., Silveira C.R., Bayeux B.M., Dias E.A., Monteiro F.M., Accorsi M., Lopes R.N., Baruselli P.S. Plasma anti-mullerian hormone: an endocrine marker for in vitro embryo production from Bos taurus and Bos indicus donors. Domestic Animal Endocrinology, 2014, 49: 96-104 ( ).
- DOI: 10.1016/j.domaniend.2014.07.002
- Gimenes L.U., Ferraz M.L., Fantinato-Neto P., Chiaratti M.R., Mesquita L.G., Sá Filho M.F., Meirelles F.V., Trinca L.A., Rennó F.P., Watanabe Y.F., Baruselli P.S. The interval between the emergence of pharmacologically synchronized ovarian follicular waves and ovum pickup does not significantly affect in vitro embryo production in Bos indicus, Bos taurus, and Bubalus bubalis. Theriogenology, 2015, 83(3): 385-393 ( ).
- DOI: 10.1016/j.theriogenology.2014.09.030
- Thibier M. Stabilization of numbers of in vivo collected embryos in cattle but significant increases of in vitro bovine produced embryos in some parts of the world. IETS Embryo Transfer Society Newsletter, 2004, 22: 12-19.
- Rubin K.C.P., Pontes J.H.F., Nonato-Junior I., Ereno-Junior J.C., Pansard H., Seneda M. Influência do grau de sangue Nelore na produção in vivo de oócitos. Acta Scientiae Veterinariae, 2005, 33: 183.
- Batista E.O.S., Macedo G.G., Sala R.V., Ortolan M., Sá Filho M.F., Del Valle T.A., Jesus E.F., Lopes R., Rennó F.P., Baruselli P.S. Plasma antimullerian hormone as a predictor of ovarian antral follicular population in Bos indicus (Nelore) and Bos taurus (Holstein) heifers. Reproduction in Domestic Animals, 2014, 49(3): 448-452 ( ).
- DOI: 10.1111/rda.12304
- Bols P.E., Leroy J.L., Vanholder T., Van Soom A. A comparison of a mechanical sector and a linear array transducer for ultrasound-guided transvaginal oocyte retrieval (OPU) in the cow. Theriogenology, 2004, 62(5): 906-914 ( ).
- DOI: 10.1016/j.theriogenology.2003.12.016
- Ward F.A., Lonergan P., Enright B.P., Boland M.P. Factors affecting recovery and quality of oocytes for bovine embryo production in vitro using ovum pick-up technology. Theriogenology, 2000, 54(3): 433-446 (
- DOI: 10.1016/s0093-691x(00)00360-5)
- Manik R.S., Singla S.K., Palta P. Collection of oocytes through transvaginal ultrasound-guided aspiration of follicles in an Indian breed of cattle. Animal Reproduction Science, 2003, 76(3-4): 155-161 (
- DOI: 10.1016/s0378-4320(02)00241-5)
- Sasamoto Y., Sakaguchi M., Katagiri S., Yamada Y., Takahashi Y. The effects of twisting and type of aspiration needle on the efficiency of transvaginal ultrasound-guided ovum pick-up in cattle. Journal of Veterinary Medical Science, 2003, 65(10): 1083-1086 ( ).
- DOI: 10.1292/jvms.65.1083
- Bols P.E., Van Soom A., Ysebaert M.T., Vandenheede J.M., de Kruif A. Effects of aspiration vacuum and needle diameter on cumulus oocyte complex morphology and developmental capacity of bovine oocytes. Theriogenology, 1996, 45(5): 1001-1014 (
- DOI: 10.1016/0093-691x(96)00028-3)
- Bols P.E., Ysebaert M.T., Van Soom A., de Kruif A. Effects of needle tip bevel and aspiration procedure on the morphology and developmental capacity of bovine compact cumulus oocyte complexes. Theriogenology, 1997, 47(6): 1221-1236 (
- DOI: 10.1016/s0093-691x(97)00102-7)
- Fayrer-Hosken R.A., Caudle A.B. The laparoscope in follicular oocyte collection and gamete intrafallopian transfer and fertilization (GIFT). Theriogenology, 1991, 36(5): 709-725 (
- DOI: 10.1016/0093-691x(91)90337-d)
- Gradela A., Esper C.R., Matos S.P.M., Lanza J.A., Deragon L.A.G., Malheiros R.M. Dominant follicle removal by ultrasound guided transvaginal aspiration and superovulatory response in Nellore cows. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 2000, 52(1): 53-58.
- Armstrong D.T., Holm P., Irvine B., Petersen B.A., Stubbings R.B., McLean D., Seamark R.F. Pregnancies and live birth from in vitro fertilization of calf oocytes collected by laparoscopic follicular aspiration. Theriogenology, 1992, 38(4): 667-678 (
- DOI: 10.1016/0093-691x(92)90029-q)
- Becker F., Kanitz W., Nurnberg G., Kurth J., Spitschak M. Comparison of repeated transvaginal ovum pick up in heifers by ultrasonographic and endoscopic instruments. Theriogenology, 1996, 46(6): 999-1007 (
- DOI: 10.1016/S0093-691X(96)00264-6)
- Santl B., Wenigerkind H., Schernthaner W., Mödl J., Stojkovic M., Prelle K., Holtz W., Brem G., Wolf E. Comparison of ultrasound-guided vs laparoscopic transvaginal ovum pick-up (OPU) in Simmental heifers. Theriogenology, 1998, 50(1): 89-100 (
- DOI: 10.1016/s0093-691x(98)00116-2)
- Baldassarre H., Currin L., Michalovic L., Bellefleur A.M., Gutierrez K., Mondadori R.G., Glanzner W.G., Schuermann Y., Bohrer R.C., Dicks N., Lopez R., Grand F.-X., Vigneault C., Blondinm P., Gourdon J., Bordignon V. Interval of gonadotropin administration for in vitro embryo production from oocytes collected from Holstein calves between 2 and 6 months of age by repeated laparoscopy. Theriogenology, 2018, 116: 64-70 ( ).
- DOI: 10.1016/j.theriogenology.2018.05.005
- Taneja M., Bols P.E., de Velde A.V., Ju J.C., Schreiber D., Tripp M.W., Yang X. Developmental competence of juvenile calf oocytes in vitro and in vivo: influence of donor animal variation and repeated gonadotropin stimulation. Biology of Reproduction, 2000; 62(1): 206-213 ( ).
- DOI: 10.1095/biolreprod62.1.206
- Lohuis M.M. Potential benefits of bovine embryo-manipulation technologies to genetic improvement programs. Theriogenology, 1995, 43(1): 51-60 (
- DOI: 10.1016/0093-691X(94)00016-N)
- Reichenbach H.D., Wiebke N.H., Modl J., Zhu J., Brem G. Laparoscopy through the vaginal fornix of cows for the repeated aspiration of follicular oocytes. Veterinary Record, 1994, 135(15): 353-356 ( ).
- DOI: 10.1136/vr.135.15.353
- Keefer C.L. Artificial cloning of domestic animals. PNAS USA, 2015, 112(29): 8874-8878 ( ).
- DOI: 10.1073/pnas.1501718112
- Bondioli K.R. Cloning of livestock by somatic cell nuclear transfer. In: Animal Biotechnology 2 /H. Niemann, C. Wrenzycki (eds.). Springer, Cham, 2018: 1-20 ( ).
- DOI: 10.1007/978-3-319-92348-2_1
- Lopukhov A.V., Singina G.N., Zinovieva N.A. Biotechnological bases of the development of cloned pig embryos. Vavilovskii Zhurnal Genetiki i Selektsii =Vavilov Journal of Genetics and Breeding, 2019, 23(5): 527-533 ( ).
- DOI: 10.18699/VJ19.521
- Sims M., First N.L. Production of fetuses from totipotent cultured bovine inner cell mass cells. Theriogenology, 1993, 39(1): 313 (
- DOI: 10.1016/0093-691X(93)90168-5)
- Vignon X., Chesné P., Le Bourhis D., Heyman Y., Renard J.P. Developmental potential of bovine embryos reconstructed with somatic nuclei from cultured skin and muscle fetal cells. Theriogenology, 1998, 49: 392 (
- DOI: 10.1016/S0093-691X(98)90745-2)
- Sermyagin A.A., Belous A.A., Konte A.F., Philipchenko A.A., Ermilov A.N., Yanchukov I.N., Plemyashov K.V., Brem G., Zinovieva N.A. Genomic evaluation of bulls for daughters' milk traits in Russian Black-and-White and Holstein cattle population through the validation procedure. Sel'skokhozyaistvennaya Biologiya (Agricultural Biology), 2017, 52(6): 1148-1156 ( ).
- DOI: 10.15389/agrobiology.2017.6.1148eng
- Серов О.Л. Трансгенные животные: фундаментальные и прикладные аспекты. Вавиловский журнал генетики и селекции, 2013, 17(4/2): 1055-1064.
- Zinovieva N.A., Volkova N.A., Bagirov V.A., Brem G. Transgenic farm animals: the status of research and prospects. Russian Journal of Genetics: Applied Research, 2016, 6(6): 657-668 ( ).
- DOI: 10.1134/S2079059716060101
- Gordon J.W., Scangos G.A., Plotkin D.J., Barbosa J.A., Ruddle F.H. Genetic transformation of mouse embryos by microinjection of purified DNA. PNAS USA, 1980, 77(12): 7380-7384 ( ).
- DOI: 10.1073/pnas.77.12.7380
- Hammer R., Pursel V., Rexroad J., Wall R.J., Bolt D.J., Ebert K.M., Palmiter R.D., Brinster R.L. Production of transgenic rabbits, sheep and pigs by microinjection. Nature, 1985, 315: 680-683 ( ).
- DOI: 10.1038/315680a0
- Brem G., Brenig B., Goodman H.M., Selden R.C., Graf F., Kruff B., Springman K., Hondele J., Meyer J., Winnacker E.-L. Production of transgenic mice, rabbits and pigs by microinjection into pronuclei. Reproduction in Domestic Animals, 1985, 20(4): 251-252 ( ).
- DOI: 10.1111/j.1439-0531.1985.tb00423.x
- Krimpenfort P., Rademakers A., Eyestone W., van der Schans A., van den Broek S., Kooiman P., Kootwijk E., Platenburg G., Pieper F., Strijker R., de Boer H. Generation of transgenic dairy cattle using 'in vitro' embryo production. Biotechnology, 1991, 9: 844-847 ( ).
- DOI: 10.1038/nbt0991-844
- Wall R.J. Pronuclear microinjection. Cloning and Stem Cells, 2001, 3(4): 209-220 ( ).
- DOI: 10.1089/15362300152725936
- Seidel G.E. Jr. Resource requirements for transgenic livestock research. Journal of Animal Science, 1993, 71(S. 3): 26-33 ( ).
- DOI: 10.2527/1993.71suppl_326x
- Eyestone W.H. Challenges and progress in the production of transgenic cattle. Reproduction, Fertility and Development, 1994, 6(5): 647-652 ( ).
- DOI: 10.1071/rd9940647
- Laible G. Production of transgenic livestock: overview of transgenic technologies. In: Animal Biotechnology 2 /H. Niemann, C. Wrenzycki (eds.). Springer, Cham, 2018: 95-121 ( ).
- DOI: 10.1007/978-3-319-92348-2_6
- Galli C., Lagutina I., Lazzari G. Introduction to cloning by nuclear transplantation. Cloning and Stem Cells, 2003, 5(4): 223-232 ( ).
- DOI: 10.1089/153623003772032745
- Cibelli J.B., Stice S.L., Golueke P.J., Kane J., Jerry J., Blackwell C., Ponce de Leon A., Robl J.M. Cloned transgenic calves produced from non-quiescent fetal fibroblasts. Science, 1998, 280(5367): 1256-1258 ( ).
- DOI: 10.1126/science.280.5367.1256
- Chan A.W.S., Homan E.J., Ballou L.U., Burns J.C., Bremel R.D. Transgenic cattle produced by reverse-transcribed gene transfer in oocytes. PNAS USA, 1998, 95(24): 14028-14033 ( ).
- DOI: 10.1073/pnas.95.24.14028
- Kuroiwa Y., Kasinathan P., Choi Y.J., Naeem R., Tomizuka K., Sullivan E.J., Knott J.G., Duteau A., Goldsby R.A., Osborne B.A., Ishida I., Robl J.M. Cloned transchromosomic calves producing human immunoglobulin. Nature Biotechnology, 2002, 20(9): 889-94 ( ).
- DOI: 10.1038/nbt727
- Hofmann A., Zakhartchenko V., Weppert M., Sebald H., Wenigerkind H., Brem G., Wolf E., Pfeifer A. Generation of transgenic cattle by lentiviral gene transfer into oocytes. Biology of Reproduction, 2004, 71(2): 405-409 ( ).
- DOI: 10.1095/biolreprod.104.028472
- Richt J.A., Kasinathan P., Hamir A.N., Castilla J., Sathiyaseelan T., Vargas F., Sathiyaseelan J., Wu H., Matsushita H., Koster J., Kato S., Ishida I., Soto C., Robl J.M., Kuroiwa Y. Production of cattle lacking prion protein. Nature Biotechnology, 2007, 25: 132-138 ( ).
- DOI: 10.1038/nbt1271
- Yu S., Luo J., Song Z., Ding F., Dai Y., Li N. Highly efficient modification of beta-lactoglobulin (BLG) gene via zinc-finger nucleases in cattle. Cell Research, 2011, 21(11): 1638-1640 ( ).
- DOI: 10.1038/cr.2011.153
- Liu X., Wang Y., Guo W., Chang B., Liu J., Guo Z., Quan F., Zhang Y. Zinc-finger nickase-mediated insertion of the lysostaphin gene into the beta-casein locus in cloned cows. Nature Communications, 2013, 4(1): 2565 ( ).
- DOI: 10.1038/ncomms3565
- Proudfoot C., Carlson D.F., Huddart R., Long C.R., Pryor J.H., King T.J., Lillico S.G., Mileham A.J., McLaren D.G., Whitelaw C.B., Fahrenkrug S.C. Genome edited sheep and cattle. Transgenic Research, 2015, 24(1): 147-153 ( ).
- DOI: 10.1007/s11248-014-9832-x
- Wu H., Wang Y., Zhang Y., Yang M., Lv J., Liu J., Zhang Y. TALE nickase-mediated SP110 knock-in endows cattle with increased resistance to tuberculosis. PNAS, 2015, 112(13): E1530-E1539 ( ).
- DOI: 10.1073/pnas.1421587112
- Yum S.Y., Lee S.J., Kim H.M., Choi W.J., Park J.H., Lee W.W, Kim H.J., Bae S.H., Lee J.H., Moon J.Y., Lee J.H., Lee C.I., Son B.J., Song S.H., Ji S.M., Kim S.J., Jang G. Efficient generation of transgenic cattle using the DNA transposon and their analysis by next-generation sequencing. Scientific Reports, 2016, 6: 27185 ( ).
- DOI: 10.1038/srep27185
- Gao Y., Wu H., Wang Y., Liu X., Chen L., Li Q., Cui C., Liu X., Zhang J., Zhang Y. Single Cas9 nickase induced generation of NRAMP1 knock-in cattle with reduced off-target effects. Genome Biology, 2017, 18(1): 13 ( ).
- DOI: 10.1186/s13059-016-1144-4
- Bosch P., Forcato D.O., Alustiza F.E., Alessio A.P., Fili A.E., Olmos Nicotra M.F., Liaudat A.C., Rodríguez N., Talluri T.R., Kues W.A. Exogenous enzymes upgrade transgenesis and genetic engineering of farm animals. Cellular and Molecular Life Sciences, 2015, 72: 1907-1929 ( ).
- DOI: 10.1007/s00018-015-1842-1
- Сингина Г.Н., Волкова Н.А., Багиров В.А., Зиновьева Н.А. Криобанки соматических клеток как перспективный способ сохранения генетических ресурсов животных. Сельскохозяйственная биология, 2014, 6: 3-14 ( ).
- DOI: 10.15389/agrobiology.2014.6.3rus


