Effects of ultrasonic treatment on protein extractability during complex processing of sunflower seeds
Автор: Shaginova L. O., Zabodalova L. A., Demianenko T. F., Domoroshchenkova M. L., Krylova I. V.
Журнал: Вестник Воронежского государственного университета инженерных технологий @vestnik-vsuet
Рубрика: Пищевая биотехнология
Статья в выпуске: 2 (100) т.86, 2024 года.
Бесплатный доступ
In this study sunflower seed cake produced on a screw press by cold pressing of dehulled sunflower seeds of confectionery-type sunflower was used as a raw material for obtaining of sunflower protein preparations. Ultrasonic treatment of the phenol-free cake was applied for improvement of extractability of proteins after the removal of phenolic compounds by treatment of dehulled sunflower press cake with an aqueous ethyl alcohol solution. Sonification was accomplished by the generator at ultrasound frequency of 22.00 ± 1.65 kНz with a duration of ultrasonic treatment from 5 to 15 minutes. Extraction of proteins was carried out in a mild alkaline medium with 0.1 % sodium bicarbonate solution with a hydro module of 1: 5 and temperature of 45 ± 2° C. Protein and dry matter content of protein extracts proportionally increased after 5-12 minutes of ultrasonic treatment with the formation of homogeneous protein extracts stable to solid phase separation and characterized by a lighter colour in comparison with the control sample not subjected to ultrasonic processing. However, after 15 minutes of ultrasonic treatment protein extracts lost uniformity with sedimentation of a solid fraction. Studies demonstrated that application of ultrasonic treatment prior protein extraction resulted in a growth of dry matter and crude protein content of sunflower protein extracts correspondently to the duration of sonification. This effect might be beneficial for an increase of protein yields in processes of manufacturing of light-coloured sunflower protein concentrates and isolates and for manufacturing of functional protein drinks from sunflower seeds. Additionally, it has been shown that sonication of sunflower protein solutions obtained after extraction of protein substances with 0.1 % NаНСО3 solution and removal of insoluble residue significantly improved their sedimentation stability and resulted in a lighter colour of resulted protein solutions in comparison with a conventional treatment by a high-speed disperser. Such treatment might be used in processes of manufacturing of dairy milk analogues from sunflower seeds.
Sunflower seeds, sunflower protein, protein extraction, phenolic compounds, ultrasonic treatment
Короткий адрес: https://sciup.org/140306936
IDR: 140306936 | УДК: 640 | DOI: 10.20914/2310-1202-2024-2-255-261
Текст научной статьи Effects of ultrasonic treatment on protein extractability during complex processing of sunflower seeds
Development of methods of production of edible protein supplements from non-traditional plant raw materials is one of the important objectives of avoiding protein deficiency, reducing dependence on proteins of animal origin and improving the structure of human nutrition. Replacing animal protein with vegetable protein in human diets is regarded as a perspective solution for achievement of more sustainable global agri-food systems and it is essential for improving the sustainability of diets [1]. According to the publication of "Agroinvestor" magazine dated 03/27/2021, by 2035 the market of products derived from plant raw materials will account 11% of the total protein market. It is forecasted that alternative plant products will have the fastest development in Russia which will allow Russia to strengthen its position as the largest agricultural supplier of plant products [2].
Nowadays all over the world research and commercial production of meat and dairy analogues are being actively developing using vegetable proteins from soybeans, peas, wheat, oats and other crops. Sunflower seed occupies a more modest position in the world vegetable protein resources compared to other protein reach crops. But for Russia this oilseed is a promising raw material for production of vegetable protein ingredients and products. Sunflower is the main oilseed crop cultivated in Russia. Sunflower seed production in the country has grown significantly over the past 20 years from 2.7 million MT in 2001 to a record crop of 15.4 million MT in 2019.
The potential of sunflower ( Helianthus annuus L .) as a raw material for obtaining of vegetable proteins is significantly underestimated. The bulk of sunflower seed is crushed at oil mills with production of vegetable oil and fodder cakes and meal. Food utilization is mainly limited to sales of packaged sunflower seeds and kernels through retail networks and by usage of dehulled sunflower seeds and flour at confectionery and bakery enterprises.
Nitrogen-containing compounds rank on the second position after lipids in composition of
This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License
sunflower seeds. Seeds of modern sunflower varieties and hybrids contain on average 45–52% fats and 17– 22% proteins. Sunflower seed storage proteins have up to 70–80% of globulins and a high biological value.
Many research papers are devoted to development of methods for obtaining of protein products from sunflower seed [3–12]. The main problems for commercial production of sunflower proteins are connected with high fiber content of sunflower cakes and meals and with the presence of phenolic compounds. Polyphenols can oxidize during storage and processing especially during conventional alkaline or salt protein extraction. Besides it could decrease nutritional and functional properties of sunflower protein preparations. [13–18]. To devoid this problem we’ve introduced the stage of removing phenolic compounds with an aqueous solution of ethyl alcohol into the scheme of complex processing of sunflower seeds [19]. But treatment of sunflower raw material with a hydrophobic solvent resulted in a denaturation of proteins with a decrease of solubility of protein substances. It limits protein extractability with water and with aqueous solutions of electrolytes and will negatively impact on sunflower protein isolates and concentrates yields and functionality and on protein content in dairy milk analogues from sunflower seeds.
To develop an effective method of production of sunflower protein preparations and products it is important to use specially dehulled high-protein seed and deoiled feedstock as a raw material and to intensify the process of protein extraction after removal of phenolic compounds.
Physical methods are perspective for improvement of extractability of target components, such as high pressure treatment, sonification, exposure to microwaves. The main advantage of these methods is their environmental friendliness, avoiding of organic solvents and aggressive chemical media, absence of waste waters and et. These methods can increase the protein yield up to 80% [20]. Today a special attention is paid to ultrasonic processing which is absolutely harmless to the environment, requires less processing impact, less maintenance costs, and is easy to install and use [21].
In the current study confectionery-type sunflower seeds are used as a raw material to produce protein preparations. These seeds are characterized by a lower oil content compared to oil varieties of sunflower seeds and can be easier dehulled. An environmentally friendly mechanical pressing method is used to extract the oil. Removal of phenolic compounds is performed by treating the press cake with an aqueous ethyl alcohol solution. The experiments are performed to investigate effects of sonification on extractability of proteins from dehulled and mechanically deoiled sunflower seed after ethyl alcohol treatment. Additionally, the effect of ultrasonic
Materials and Methods
Two commercial batches of confectionerytype sunflower seeds with different protein content were selected for experiments. Two samples of dehulled seeds were obtained by mechanical separation and removal of hulls. Crude protein, fat and moisture content of dehulled seed samples # 1.1. and # 2.1. are presented in Table 1.
Table 1. Chemical composition of dehulled sunflower seed samples
Parameters Sample #1.1 Sample #2.1
Moisture and volatiles, % 4,13 ± 0,05 4,47 ± 0,03
Crude protein (Nх6.25), % m.f.b. * 26,79 ± 0,03 23,33 ± 0,02
Crude fat (ether extr.), % m.f.b. 44,42 ± 0,14 49,67 ± 0,15
* m.f.b. – moisture free basis
Source : Compiled by the authors
Sunflower oil was extracted by cold pressing of dehulled seeds at Akita jp AKJP 700 oil press professional screw oil press with capacity of 18 to 20 kg/h. Samples #1.2 and #2.2 of dehulled sunflower press cake with different crude protein content were obtained. The chemical composition of the samples is presented in Table 2.
Table 2 Chemical composition of dehulled sunflower press cake samples
Table 2.
Chemical composition of dehulled sunflower press cake samples
Parameters Sample #2.1 Sample #2.2
Moisture and volatiles, % 7,16 ± 0,03 7,48 ± 0,04
Crude protein (Nх6.25), % m.f.b. 46,63 ± 0,05 39,16 ± 0,02 Crude fat (ether extr.), % m.f.b. 9,5 ± 0,08 9,34 ± 0,06
Source : Compiled by the authors
For removal of phenolic compounds sunflower cake samples #1.2 and #2.2 were extracted by 70% aqueous ethyl alcohol solution followed by washing of a solid residue with acidified water [19]. Then 0.1% NаНСО 3 aqueous solution was used for extraction of proteins in an alkaline medium at a ratio of 1: 5 and temperature of 45 + 2 °C for 30 minutes and a stirring speed of 1,000 rpm. Soluble protein extracts were separated from insoluble cake residues by centrifugation at 2,000 rpm.
Ultrasonic treatment was applied at two different stages of complex processing of sunflower seeds.
-
1. Ultrasonic treatment of de-phenolic sunflower press cake samples prior extraction of protein substances.
-
2. Ultrasonic treatment of protein solution after removal of insoluble cake residue.
Ultrasonic treatment prior extraction of proteins by sodium bicarbonate solution was carried out at I100–6/4 ultrasonic generator (manufactured by LLC "Ultrasonic technology – INLAB", Russia) at a working ultrasound frequency of 22.00 ± 1.65 kНz; power consumption 100 W with a duration of ultrasonic treatment from 5 to 15 minutes (samples #1.3 and #2.3). A sample of the protein extract not subjected to ultrasonic treatment was used as a control (0 minutes).
Impact of sonification on the sedimentation stability of protein solutions was carried out by ultrasonic treatment for 4 minutes at a working ultrasound frequency of 22.00 ± 1.65 kНz (sample #1.4) and compared with processing of protein solution in a laboratory disperser Т25 digital ULTRA-TURRAX® , IKA, Germany at 13.5 thousand rpm for 30 minutes (sample #1.5).
To avoid overheating of materials during sonification and to maintain the optimum temperature, an ice bath was used in both cases of ultrasound treatment.
The protocol followed for the complex processing of sunflower seed including ultrasonic treatment stages is illustrated in figure 1.
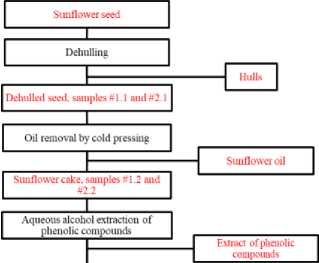
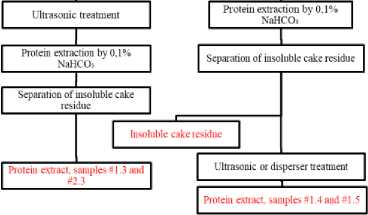
Figure 1. Flow diagram of stages of complex processing of sunflower seed. Source : Compiled by the authors
Dry matter content, crude protein and crude fat content were analyzed in sunflower raw materials, control and experimental samples.
Dry matter contents were determined gravi-metrically at 105 °C following the protocol of the Russian state standard GOST 13979.1.
Crude protein contents of samples were calculated based on their nitrogen content (Nх6.25) as determined by Kjeldahl method using Protein/ Nitrogen analyzer KjelFlex K-360 of BUCHI following the protocol of the Russian state standard GOST 13496.4.
Crude fat contents of samples were determined by extraction of oil from samples by diethyl ether using the Soxhlet apparatus and method following the protocol of the Russian state standard GOST R 53153.
Sedimentation stability and colour of resulting protein solutions were evaluated visually.
Distribution of fat globules in protein solutions before and after ultrasonic treatment was observed at Axio Lab. А1 laboratory microscope (manufactured by Carl Zeiss, Germany) with magnification 63х.
All experiments were carried out in three replicates.
Results
The effect of ultrasonic treatment of dephenolic sunflower press cake samples that was applied prior the extraction of protein substances with 0.1% NаНСО 3 solution on dry matter and crude protein content of protein extracts (samples #1.3 and #2.3) is shown in Table 3.
The growth of crude protein and dry matter content in protein extracts produced with ultrasonic treatment expressed as a percent to the corresponding parameters of the control extract without ultrasonic treatment is shown in Fig. 2.1 and 2.2. The content of crude protein and dry matter in the control (0 minutes) sample is taken as 100%.
Table 3.
Effect of ultrasonic treatment on crude protein and dry matter content of protein extracts in sodium bicarbonate solution
|
Duration of treatment |
Crude protein, % |
Dry matter content, % |
||
|
Sample #1.3 |
Sample #2.3 |
Sample #1.3 |
Sample #2.3 |
|
|
Control – 0 min. |
2,23 ± 0,005 |
1,57 ± 0,002 |
2,56 ± 0,014 |
2,01 ± 0,002 |
|
5 minutes |
2,64 ± 0,021 |
1,65 ± 0,005 |
2,9 ± 0,07 |
2,19 ± 0,04 |
|
10 minutes |
2,88 ± 0,003 |
1,71 ± 0,004 |
3,3 ± 0,05 |
2,21 ± 0,08 |
|
12 minutes |
- |
1,98 ± 0,006 |
- |
2,77 ± 0,09 |
|
15 minutes |
3,69 ± 0,006 |
3,05 ± 0,004 |
4,5 ± 0,02 |
4,28 ± 0,04 |
|
Source : Compiled by the authors |
||||
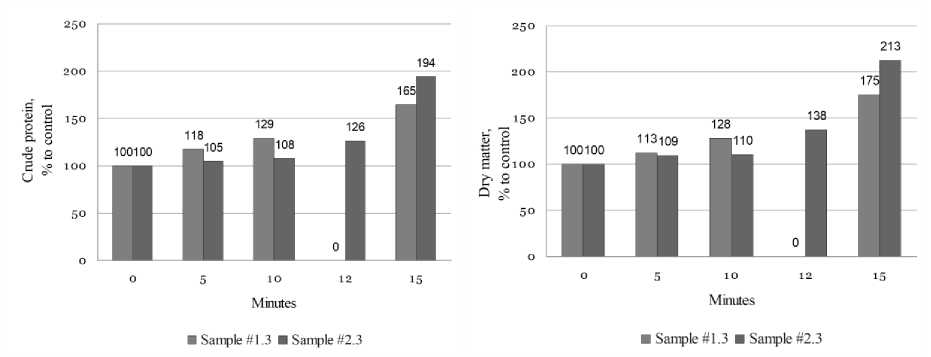
Figure 2. Growth of crude protein and dry matter content in protein extracts after ultrasonic treatment in percent to corresponding control sample levels expressed as 100%. Source : Compiled by the authors
The effect of the duration of ultrasonic treatment of sample #1.3 on the appearance of the protein extracts is shown in Figure 3.

Figure 3. Photo of protein extracts in 0,1% NаНСО 3 solution without ultrasonic treatment (control) and with duration of ultrasonic treatment of de-phenolic sample #1.2 during 5, 10 and 15 minutes. Source : Taken by the authors
The effect of ultrasonic treatment on the distribution and size of fat globules in sample #1.3 under a microscope in comparison with the control protein extract without ultrasonic treatment is shown in Figure 4.
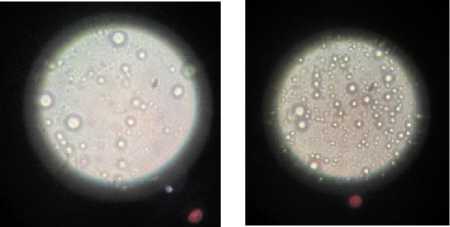
Figure 4. Photo of protein extracts taken with microscope magnification. Left – control sample without ultrasonic treatment. Right – sample after sonification. Source : Taken by the authors
The effect of ultrasonic treatment of protein solutions obtained after extraction of protein substances with 0.1% NаНСО 3 solution and separation of insoluble residue on their appearance in comparison with processing in a disperser is shown in figure 5.

Figure 5. Photo of protein solutions after treatment by 2 – ultrasound generator (sample #1.4) and 1 – disperser (sample #1.5)
Discussion
The results of experiments demonstrate that introduction of the sonification stage prior the extraction of protein substances from the material treated with alcohol the content of dry matter and crude protein in protein extracts (samples #1.3 and #2.3) grows with an increase of sonification time (Table 3). During the first 10 minutes of sonification the protein concentration in the extracted solution proportionally increases by 29% for sample #1.3 and by 8% for sample #2.3, the concentration of dry substances in the extracts increases by 28% for sample #1.3 and 10% for sample #2.3 respectively (Figure 2).
Protein extracts obtained from de-phenolic sunflower press cake sample #1.2 after sonification for 15 minutes quickly lost uniformity with sedimentation of a solid fraction while such effects didn’t occur during sonication for 10 minutes or less. In experiments with de-phenolic sample #2.2 the effect of the additional sonification time of 12 minutes was studied. It was observed that such treatment didn’t cause phase separation of the resulted protein extract. After sonification for 15 minutes, the crude protein content in the extracted solutions obtained from
Шагинова Л.О. и др. Вестник ВГУИТ, 2024, Т. 86, №. 2, С. both samples of de-phenolic sunflower press cakes increased sharply – by 65% (sample #1.3) and 94% (sample #2.3) compared to the crude protein content in the control extracts without sonification (Figure 2). However, these protein extracts were unstable forming a precipitate during the day storage at a room temperature. Perhaps 15 minutes sonification will have a positive effect on yields of protein isolates and concentrates but may lead to the loss of their functional properties. Protein extracts after sonication had a lighter colour in comparison with the control sample (Figure 3). Apparently, after ultrasonic treatment during time up to 10–12 minutes there is a change in the size of the fat globules and formation of a more uniform distribution of protein and fat molecules preventing coalesation. Microscopic examination of the structure of the protein solution showed a significant reduction the size of fat globules and a more uniform distribution after sonication (Figure 4), which positively influenced the system's sedimentation stability.
After 15 minutes of sonification more drastic structural changes could occur with the loose of the system stability and formation of a precipitate. We consider that for methods of production of functional protein drinks with an increased protein content from sunflower seeds the sonification stage should be less than 15 minutes to prevent separation of the product during storage which will negatively affect the consumer perception of the sunflower drink.
In the 2nd series of experiments introduction of the 4 minutes sonication treatment of the protein solutions obtained after extraction of protein substances with 0.1% NаНСО3 and removal of cake insoluble residue resulted in a significant improvement in their sedimentation stability and appearance compared with a conventional treatment in a disperser during 30 minutes. In protein solutions treated with ultrasound a precipitate was formed on the 2nd day of storage compared to the formation of the precipitate during the 1st day in samples treated in a disperser stored at the same storage conditions in a refrigerator at a temperature of 4 ± 2 °C. Another positive result was that when the samples were sonicated, the protein solution had a lighter colour compared to the treatment in a disperser (Figure 5). These effects are very promising for development of dairy milk analogues from sunflower seeds with introduction of an ultrasound treatment.
Conclusion
The experiments have demonstrated that introduction of the stage of ultrasonic treatment after alcohol extraction of phenolic compounds from the dehulled sunflower press cake seed in complex processing of confectionery-type sunflower seeds significantly increases the protein extractability with 0.1% NаНСО 3 solution. The growth of crude protein extractability after sonication will have a positive effect on protein yields in conventional methods of production of sunflower protein concentrates and isolates by alkaline and salt extraction. In addition, sonication is a promising treatment for increasing of the protein concentration in functional protein drinks from sunflower seeds.
Sonication of sunflower protein solutions improves their sedimentation stability and forms a lighter coloured finished product compared to conventional treatment in a disperser.
Список литературы Effects of ultrasonic treatment on protein extractability during complex processing of sunflower seeds
- Aiking H., de Boer J. The next protein transition. Trends in Food Science and Technology. 2020. vol. 105. pp. 515-522.
- Agroinvestor. Available at: https://www.agroinvestor.ru/markets/news/35506alternativnye-moloko-i-myaso-mogut-zanyat11rynka-belkov-k2035godu
- Taha F.S., Abbasy M., El Nockrashy A.S., Shoeb Z.E. Countercurrent extraction and isoelectric precipitation of sunflower seed protein isolates. Journal of the Science of Food and Agriculture. 1981. vol. 32. no. 2. pp. 166-174.
- Parrado J., Bautista J., Machado A. Production of soluble enzymic protein hydrolyzate from industrially defatted nondehulled sunflower meal. Journal of agricultural and food chemistry. 1991. vol. 39. no. 3. pp. 447-450.
- González-Pérez S., Merck K.B., Vereijken J.M. et al. Isolation and characterization of undenatured chlorogenic acid free sunflower (Helianthus annuus) proteins. Journal of Agricultural Food Chemistry. 2002. vol. 50. no. 6. pp. 1713-1719.
- Ivanova P., Chalova V., Koleva L., Pishtiyski I. et al. Optimization of protein extraction from sunflower meal produced in Bulgaria. Bulg. J. Agric. Sci. 2012. vol. 18. pp. 153-160.
- Shirokoryadova O.V. et al. Sunflower meal is an economically promising raw material for the production of food protein and carbohydrate products Izvestiya vuzov. Food technology. 2009. vol. 5. no. 6. pp. 45-48.
- Pickardt C., Hager T., Eisner P., Carle R. et al. Isoelectric protein precipitation from mild-acidic extracts of de-oiled sunflower (Helianthus annuus L.) press cake. Eur Food Res Technol. 2011. vol. 233. pp. 31-44.
- Voichenko O.N. et al. Evaluation of sunflower seed processing products as alternative sources of dietary protein. Izvestiya vuzov Food technology. 2013. vol. 4. no. 334. pp. 88-90.
- Shchekoldina T.V. Technology for production of protein-containing raw materials from the products of sunflower seeds. Polythematic online scientific journal of Kuban State Agrarian University. 2015. vol. 109. no. 05. pp. 360-378.
- Domoroshchenkova M.L., Demianenko T.F., Krylova I.V. Prospects for obtaining proteins from sunflower meal. Oils and Fats. 2016. vol. 5-6. pp. 22-23.
- Ovsiannikova O.V., Frantseva T.P. Development of technology for obtaining food protein products from sunflower seeds. Saint-Petersburg, Lan, 2017.
- Cater C.M., Gheyasuddin S., Mattil K.F. The effect of chlorogenic, quinic and caffeic acids on the solubility and color of protein isolates, especially from sunflower seed. Cereal Chem. 1972. vol. 49. pp. 508-514.
- Vedernikova E.I. Phenolic compounds of sunflower protein isolates. Applied Biochemistry and Microbiology. 1974. vol. 10. no. 06. pp. 897-905.
- Sabir M.A., Sosulski F.W., Finlayson A.J. Chlorogenic acid-protein interactions in Sunflower. J. Agric. Food Chem. 1974. vol. 22. no. 4. pp. 575-578.
- Leung J., Fenton T.W., Clandinin D.R. Phenolic components of sunflower flour. J. Food Sci. 1981. vol. 46. no. 5. pp. 1386-1388.
- Sripad G., Prakash V., Narasinga Rao M.S. Extractability of polyphenols of sunflower seed in various solvents. J. Biosci. 1982. vol. 4. no. 2. pp. 145-152.
- Weisz G.M., Kammerer D.R., Carle R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLCDAD/ESI-MSn. Food Chem. 2009. vol. 115. no. 2. pp. 758-765.
- Shaginova L.O., Krylova I.V., Demianenko T.F., Domoroshchenkova M.L. Research of the process of obtaining protein preparation from sunflower seeds for food industry. New technologies. 2021. vol. 17. no. 3. pp. 41-50.
- Pojić M., Mišan A., Tiwari B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends in Food Science and Technology. 2018. vol. 75. pp. 93-104.
- Rahman M.M., Lamsal B.P. Ultrasound-assisted extraction andmodification of plant-based proteins: Impact onphysicochemical, functional, and nutritionalproperties. Compr Rev Food Sci Food Saf. 2021. vol. 20. pp. 1457-1480.


