Enzyme complex of wheat as new biosensor for glutamate determination
Автор: Kudiyarova Zh. S., Kerymkulova A.R., Lesova Zh.T., Bukenova E.A., Gilmanov M.K.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Естественные науки
Статья в выпуске: 4 (100), 2013 года.
Бесплатный доступ
Malate dehydrogenase-glutamate oxaloacetate aminotransferase (MDh-GOT) enzyme complex (the EC) was isolated and purified from wheat seeds. It was developed the effective method of purification of EC by using of ion-exchange chromatography and gel-chromatography methods. MDh-GOT consists of two heterological subunits. The essential feature of the EC is the irreversibility of its catalyzed reactions. Michaelis constants of the EC MDh-GOT to malate, glutamate and NAD were investigated. Good stability, high sensitivity and short time for analysis (1-2 minutes) of the EC to glutamate make it very convenient for determination of concentration of glutamate.
Enzyme complex (ec), malate dehydrogenase-glutamate oxaloacetate aminotransferase (mdh-got), glutamate
Короткий адрес: https://sciup.org/140205010
IDR: 140205010 | УДК: 57.03
Текст научной статьи Enzyme complex of wheat as new biosensor for glutamate determination
It’s well known that glutamate has high neurotoxic properties [1]. Especially it’s very dangerous to use for children till 3 years old. It’s using as food stuff for children lead to damaging of their brain system [2]. In this reason the European Commissions had forbidden the content of glutamate in children food stuff. In the same time glutamate is often added to many food stuffs for giving meet tastes. In this reason it’s very important to determine the exact quantity of glutamate in food stuffs. The determination of exact quantity of glutamate in blood and in urine of pregnant women is necessary for diagnostic of several diseases in pathological states. In this reason there is a high necessity of the development of the new biosensor for exact and reliable determination of glutamate quantity. Existing methods of determination of glutamate concentration is based on using of glutamate dehydrogenase from bovine liver. However this enzyme very unstable and easy changes its activity from various ions and ligands.
Materials and methods
Materials for isolation of the enzyme complex malate dehydrogenase (MDh) and glutamate oxaloacetate aminotransferase (GOT) were dry seeds of spring wheat cultivar "Steklovidnaya-24" (Triticum aestivum). For isolation of the EC MDh-GOT the dry seeds of cereals were milled on laboratory mill then we obtained the floor by sieving. Then 10 grams of floor of wheat were homogenized in 0,05М tris-HCl buffer, рН 7,5 taken in the ratio of 1:4, (weight to volume) in porcelain mortar. Then homogenate was centrifuged at 10 000
х g during 10 minutes. Obtained supernatant was purified from low-molecular substances and by gelchromatography on a column with Sephadex G-50, the size 3,5 x 20сm. Then the fractions containing activity of the EC were put on a column with DEAE-cellulose type DE-52 size 5 х 2 cm, Vattman (England), the both columns were pre-equilibrated by the 0,05М tris-HCl buffer, pH 7,4. MDh-GOT was eluted by 0.1M NaCl solution which was dissolved in the same buffer.
Then fraction containing the EC MDh- GOT activity was purified by gel - chromatography on a column with Sephacryl S-300, the size of a column 2 х 95сm. The optical measurement was carried out by spectrophotometer Ultrospec-1100 pro, (Bioscience, Amersham, UK) [3]. Determination of protein quantity is carried out by microbiuret Bailey methods [4]. We developed the new spectrophotometric method of determination of EC MDh-GOAT. The reaction mixture for determination of the EC activity contains 1,1 mМ NAD, 12 mМ malate and 87 mМ of sodium glutamate. The total volume of reaction mixture is equal 2 ml by adding of 0,05 М tris-glycine buffer, рН 7,7. The activity of EC was determined during 1 minute. The activity of EC expressed as formed NADH in 1 minute per 1 mg of protein. The chromatography elution control was carried out by Uvicord SU, 2238, LRB. (Pharmacia, Sweden) [5].
Results and discussions
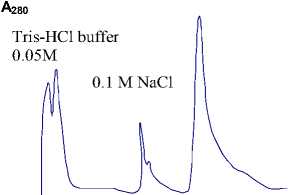
The results of separation of the EC MDh-GOAT by ion-exchange chromatography are represented at figure 1(a). The EC was eluted by 0,1М NaCl in the same buffer.
-0,2 0
0.5M NaCl
1,6
1,4
1,2
0,8
0,6
0,4
0,2
50 100 150
1,2 A 280 1
0,8
0,6
0,4
0,2
50 100
20 m 0 in 0
150 min
b
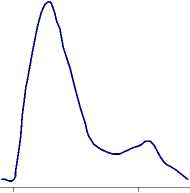
Fig.1 - Chromatography of cell-free extract from wheat variety “Kazakhstanskaya-10” of stage of the EC purification: a) on column with DEAE-cellulose; b) on column with Sephacryl S-300.
Fractions containing the EC MDh- GOAT activity then were separated by gel- chromatography on a column with Sephacryl S-300. Results of chromatographic purification are presented in figure 1(b). The fraction of the EC MDh- GOT was eluted in the second peak. The purification of the EC has shown that the EC is single, stable and strong protein complex which does not dissociate under the separation conditions. The purified EC does not contain any GDh and MDh activity as impurities.
Basic physical and chemical properties of the EC from wheat were investigated. We study EC by SDS electrophoresis by Laemmly (1970) [6]. Electrophoresis was carried out with presence of protein-markers with known molecular masses. Results are presented in figure 2.
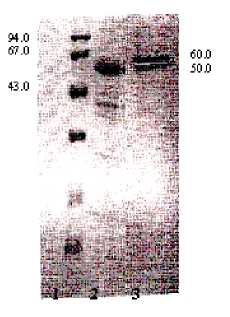
Fig.2 - SDS - electrophoresis of the enzyme complex (EC) from wheat: 1-protein-tap; 2 -the EC before FPLC; 3 - EC after FPLC. Zones of proteins markers: phosphorylase 94 kDa; bovine serum albumin 67 kDa; egg albumin 43 kDa.
Determination of molecular masse has shown that the EC consists of two heterologic subunits with molecular masses of 50 kDa and
60 kDa accordingly.
For understanding of nature of the EC first of all it was necessary to study the products of reactions catalyzed by the EC. The product of reactions was determined by enzyme method. After 5 minutes of incubation the reaction mixture was boiled during 35 min and it was filtered trough yellow paper filter. Then we determined products of reactions by using of commercial preparations of the next enzymes: glutamate dehydrogenase (GDh) from bovine liver (Sigma), MDh (Sigma) and L-aspartate aminotransferase (Sigma) [6]. In reaction mixture were found next products: 2-oxoglutarate by GDh and aspartate by L-aspartate aminotransferase. What was surprisingly is we did not find any traces of oxaloacetate in products mixture. This means that active centers of MDh and GOT of the EC located very close to each other and formed oxaloacetate is not able to leave catalytic centers of the EC. Oxaloacetate immediately transfers to aspartate by transamination reaction with glutamate. This phenomenon explains the irreversibility of reaction that catalyzed by the EC MDh-GOT as oxaloacetate could not accumulate. Thus we conclude that the EC catalyzes next consequence of reactions. First of all MDh of EC catalyzes the reaction of oxidation of malate to oxaloacetate with reducing of NAD to NADH. Then formed oxaloacetate immediately transaminates with glutamate by GOT of the EC with formation of 2-oxoglutarate and aspartate.
For using of the EC as biosensor it was necessary to study the kinetic properties of the EC. We carried out the experiment where we determined the activities of the EC at various concentrations of glutamate. Results of experiments are presented at figure 3.
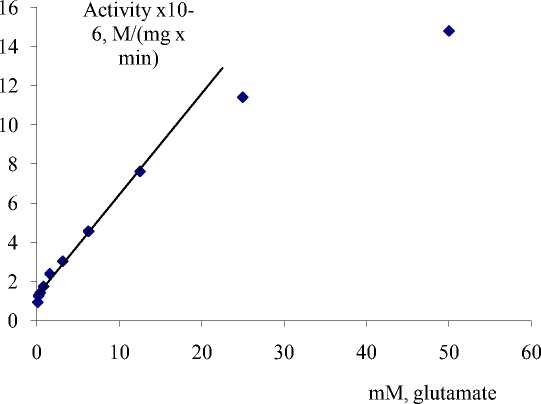
Fig.3 - The dependence of activity of EC MDh-GOAT from glutamate (RSD =1.58%).
As it shown from figure 3, EC has linear response for glutamate at concentration range from 0.1 mM till 15 mM. Then basic kinetic characteristics of the EC - the Mikhaelis constant (Km) for substrates of the EC from wheat were estimated. The following values of Km for EC of wheat - Km for glutamate 2,29∙10-3 M, for malate 1,47∙10-3 M, and for NAD 4,33∙10-4 M were determined. Kinetic characteristics of glutamate speaks that the EC is sensitive for determination of glutamate. The investigation of kinetic properties of the EC allows using it as a very effective biosensor for determination of glutamate in food stuffs and biological liquids.
We have tested the purified the EC for determination of concentration of glutamate in plasma of blood of woman with habitual loss of embryos. It was observed that in contrast the normal woman has the concentration of glutamate to 2 times less (0.227 mM for pathogenic and 0.518 mM for normal woman, RSD = 1.6%). The experiment was carried out in Kazakh Republic Centre of the Women and Child health protection. Groups with normal and pathogenic patients were observed. Each group consists of 10 people.
Conclusion
The EC MDh-GOAT is purified from wheat grains, which is cheap and readily available source.
For the first time we have discovered new enzyme complex (EC) which consists from two enzymes – malate dehydrogenase and glutamate oxaloacetate aminotransferase. The effective method of purification of the EC by using methods of ion-exchange chromatography and gel-chromatography was developed. The homogeneous preparation of the EC contains of two heterological subunits 50 and 60 kDa. It was established that the EC catalyzes the next consequence of reactions. First of all MDh of the EC catalyzes the reaction of oxidation of malate to oxaloacetate with reducing of NAD to NADH. Then formed oxaloacetate immediately transaminates with glutamate by GOT of the EC with formation of 2-oxoglutarate and aspartate. The EC in contrast of GDh catalyzes consequence of irreversible reactions which allows obtaining more reliable results of glutamate determination. The EC MDh-GOT biosensor for determination of glutamate has obvious superiority, because the properties of the EC are making it very convenient for using as biosensor. They are:
-
1. Activity of the EC is easily determines by spectrophotometer at wavelength 340 nm by detection of formation of NADH during 1-2 minute.
-
2. Irreversibility of reaction is more convenient for studying than common reverse enzyme reactions.
-
3. It is well known that animal GDh is very unstable and easy changes its activity from various ions and ligands such as ATP, ADP, Zn ions and steroid hormones. Whereas our EC is stable and mono- and divalent metal ions didn’t change the activity of the EC.
Thus we developed the new effective biosensor for wide application in medicine and food analytical chemistry.
Список литературы Enzyme complex of wheat as new biosensor for glutamate determination
- Forde, B.G., Lea, P.J., Glutamate in plants: metabolism, regulation and signalling. Journal of Experimental Botany 58 (9), 2007. -Р. 2339-2358.
- Almeida A., Heales S.J., Bolaños J.P., Medina J.M., Brain Res, 790, 1998.-Р. 209-216.
- Gilmanov M.K., Fursov O.V., Frantsev A.P. Methods of clearing and studying of enzymes of plants. -Alma-ata, 1981, pp. 91-92. (In Russian).
- Bailey J.L., Techniques in Protein Chemistry, 1962. -Р 300. London: Elsevier Publishing Co.
- Kudiyarova Zh.S., Study of key enzymes of glutamate metabolism in relation with morphogenesis and tolerance to salt and rust infections of wheat genotypes, Almaty, 2009. -93 p.
- Laemmli UK. (August 1970). "Cleavage of structural proteins during the assembly of the head of bacteriophage T4". Nature 227 (5259): 680-685.


