Foodborne illnesses caused by tissue parasitic protozoa
Автор: Rutaganira J., Glamazdin I.G.
Журнал: Вестник Воронежского государственного университета инженерных технологий @vestnik-vsuet
Рубрика: Пищевая биотехнология
Статья в выпуске: 3 (97) т.85, 2023 года.
Бесплатный доступ
Zoonotic infections caused by Sarcocystis species, known as intestinal sarcocystosis, are an example of foodborne illnesses that occur due to the consumption of contaminated meat. Zoonotic Sarcocystis parasites, specifically those hosted in cattle and swine meat, have been reported as the sole causative agents of human intestinal sarcocystosis. The infection’s symptoms include nausea, diarrhea, vomiting, and abdominal pain. Talking about zoonotic parasites goes hand in hand with discussing the parasite life cycle, animals that act as natural intermediate hosts, and humans who act as the natural definitive host for the parasite. To safeguard cattle and swine meat consumers, the government of Rwanda, through the Rwanda Inspectorate, Competition, and Consumer Protection Authority (RICA), has banned the sale of meat that has not been refrigerated for at least 24 hours and reached a temperature between 2oC and 4oC before sale in order to prevent the transmission of zoonotic and transmissible diseases. Based on various literature reports, zoonotic sarcocyst viability remains intact in this range of temperatures for a period of 24 hours. Given that the prevalence of swine coccidia in Rwanda was reported to be 55.8%, Coccidia may not only include Sarcocystis species. The established rule may not be contributing to the prevention of zoonotic sarcocystis parasite infection but it is a preventive solution for many other zoonotic parasites and pathogens. Due to the lack of research reports on zoonotic sarcocystis in Rwanda, there is limited knowledge about these parasites in the country. This lack of information may explain why the prevention and control measures taken to address zoonotic pathogen infection do not adequately address the issue of zoonotic sarcocysts. Though the parasite infection was reported to be negligible, it may induce reduced human food availability in the food production system due to the ability of the disease to be transmitted from humans to cattle and pork. These later animals' meat is prestigiously served as human food in Rwanda.
Antimicrobial activity, food and parasites, human intestinal sarcocystosis, protozoa, zoonotic parasitology, zoonotic sarcocystis species
Короткий адрес: https://sciup.org/140303229
IDR: 140303229 | УДК: 640 | DOI: 10.20914/2310-1202-2023-3-131-135
Текст научной статьи Foodborne illnesses caused by tissue parasitic protozoa
Since their initial description in 1843, over 200 species of Sarcocystis have been identified so far. Sarcocystis species organisms require two hosts (an intermediate host and a definitive host) to complete their life cycle. Apparently, herbivores act as natural intermediate hosts, while carnivores and omnivores act as natural definitive hosts. Humans and reptiles are aberrant intermediate hosts for S. nesbitti, which is responsible for muscular Sarcocystosis in humans. S. nesbitti has its definitive host in reptiles, especially snakes [4, 13].
This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License
Six Sarcocystis species, including S. cruzi, S. hominis, S. heydorni, S. bovifelis, S. hirsuta, and S. rommeli, are specific to cattle and have bovines as their intermediate hosts, with various definitive hosts. All six of them are responsible for the disease known as cattle sarcocystosis [10]. Two (S. hominis and S. heydorni) [3] of the six have zoonotic potential, and they can infect humans [2, 15, 17, 19]. S. suihominis can also infect humans, but it is primarily associated with pork [4]. Zoonoses are known to cause severe, acute, or chronic infections in humans. These infections may cause enteritis [14] infection acquired by ingesting sarcocysts transmitted by pork meat is more severe than the infection acquired by ingesting sarcocysts from cattle meat [4]. These zoonotic Sarcocystis species infect humans when diseased undercooked or raw Cattle or pork meat are consumed by humans. Though many other Sarcocystis species may infect humans but only three species (S.hominis, S.heydorni, and S.suihominis) are known to cause Intestinal Sarco-cystosis which may cause enteritis in humans who are natural definitive hosts for these parasites [14]. Diarrhea and abdominal pain were among consistent symptoms of the infection [3]. Sexual stages of the parasites which mate and produce oocyst in human intestine, excrete these oocysts containing sporocysts in human feces. Zoonotic Sarcocystis species belong to the genus of Sarcocystis , and to the sub-Kingdom of Protozoa . Sporocysts of S.sui-hominis are larger in size compared to those of S.hominis and S.heydorni [4]. The sporocysts of these three species are morphologically identical; they only differ in size and shape. Ingestion of sporocysts of the above-mentioned three zoonotic sarcocystis species from feces-contaminated feed, water, and the environment is the only route to spreading the disease and keeps the parasites circulating between humans and herbivorous animals (cattle and pork). In Rwanda and Africa in general, bovine meat and pork meat are served as prestigious human foods. Rules and regulations require the condemnation of infected parts of cattle or pig meat. In Rwanda, swine coccidia contribute to intestinal sarcocystosis by about 55.8% [16].
Objective
This article provides a review of the status and prevalence of cattle sarcocystis infection in Africa and Europe and provides important information associated with foodborne illnesses induced by the consumption of contaminated cattle and swine meat. It will also rely on the source to reproduce information on zoonotic infection prevention and control in Rwanda and parasite infection prevention and control measures in general.
post@vestnik-vsuet.ru Material and methods
References that were used include international English databases such as the National Center for Biotechnology Information (NCBI), , Google Scholar, Web of Sciences, Wiley, Elsevier, and (Source: .
Prevention and control
Anti-inflammatory drugs are currently available, which may help alleviate the symptoms of intestinal sarcocystosis. However, there is currently no vaccine or confirmed effective antiparasitic drug available. The most effective method for preventing and controlling intestinal sarcocystosis is to ensure that cattle or pork meat is cooked thoroughly for a minimum of 5 minutes at a temperature of 100°C. This process will effectively eliminate bradyzoites present in sarcocysts [14]. It is also advisable for farmers to ensure that the feed and water provided to cattle and pigs are free from sporocysts. Boiling water above 70°C for at least 1 minute or freezing the feed at -20°C for less than an hour will kill sporocysts [6, 14]. Feed can also be subjected to a pH of 10.0 for 4 days, in addition to being treated with salt for at least one day [6]. However, the parasite survived by being exposed to temperatures between 0 and 4°C and acidic conditions (pH 3.0 and 5.0) for more than 7 days while still retaining the diarrhea toxin [6].
Rwanda has implemented a ban on the sale of meat that has not been refrigerated for at least 24 hours and does not reach a temperature between 2 and 4°C prior to sale. This measure aims to prevent the transmission of zoonotic and transmissible diseases and protect consumer health by reducing the risk of contracting foodborne illnesses from meat. The ban is in accordance with legislation issued in May 2022, which aims to regulate meat enterprises in Rwanda (source: . Infective sarcocysts of zoonotic Sarcocystis species cause zoonotic Sarcocystis infection in humans, known as human intestinal sarcocysto-sis. These species include Sarcocystis hominis and Sarcocystis heydorni, which naturally infect cattle muscle tissues, and Sarcocystis suihominis, which naturally infects swine muscle tissues. According to a report by Honda and colleagues, these parasites lose their viability when frozen at -20°C for less than an hour.

Figure 1. Rwandan cattle grazing in the pasture

Figure 2. Cattle meat before subjecting to 2-4°C
Results and discussion
Zoonotic Sarcocystis species infection in human is caused by infective sarcocysts of zoonotic sarcocystis species ingested when undercooked infected bovine and swine meat muscle tissues are consumed. The parasite do not involve multiplication, from each bradyzoite within a sarcocyst, a male or a female stage are required for the development of the parasite in the human intestine [4]. Zoonotic Sarcocystis species have been reported to be food poisoning agent inducing gastro-intestinal Symptoms within 24 hours including nausea, vomiting, abdominal pain, and diarrhea [6]. Though Sarcocystis spp infection in human effect on public health was reported negligible, but it is more severe on vulnerable persons. The symptom severity depends on the amount of sarcocystis species ingested [14]. Zoonotic Sarcocystis species circulate between intermediate hosts and definitive hosts. Asexual reproduction of the parasite occurs in intermediate hosts whereas sexual reproduction occurs in definitive host. Sarcocystis hominis and S.heydorni are known to circulate between Cattle and humans to complete their life-cycle whereas S.suihominis is known to circulate between swine and Humans.
Table 1.
Zoonotic sarcocystis species and their hosts
|
Species |
Intermediate host |
Definitive host |
|
S.hominis |
Cattle |
Human |
|
S.heydorni |
Cattle |
Human |
|
S.suihominis |
Pork |
Human |
Spreading of Intestinal Sarcocystosis is mostly promoted by those who have a habit of consuming raw or undercooked meat and sometimes reside where human feces contaminate pastures. Sporocysts remain viable for many days in the pasture due to the sarcocystis parasite adaptation to the harsh environmental conditions. Once intermediate hosts graze and acquire infections, the infection in intermediate host induces abortion, weight loss, even death which can reduce cattle or swine food based in the food production system.
Figure 1 depicts the prevalence of sarcocys-tosis in cattle, as documented by various authors in their published scientific articles. In Egypt, the reported prevalence of sarcocystosis in cattle was 98.9% [9]. The reported prevalence of sarcocys-tosis in cattle in Russia was 85% [8]. The reported prevalence of sarcocystosis in cattle in Ethiopia was 82% [20]. The reported prevalence of sarcocystosis in cattle in Italy was 78.1%. The reported prevalence of sarcocystosis in cattle in the Netherlands was 76.9% [5]. The reported prevalence of sarcocysto-sis in cattle in Tunisia was 70.6% [1]. he reported prevalence of sarcocystosis in cattle in Germany was 69.6% [12]. The reported prevalence of sarcocys-tosis in cattle in Hungary was 66% [7]. The reported prevalence of coccidia in Rwanda was 55.8% [16]. However, there is insufficient data to determine the prevalence of sarcocystis infection in cattle in Rwanda. Coccidia may not only include Sarcocystis species. There have not been many reports on Sarcocystis species in Rwanda so far. From the observation, it is evident that intestinal sarcocystosis can also affect developed countries. Cases of cattle sarco-cystosis have been detected in those countries. Thus, the spread of intestinal sarcocystosis could have a significant impact on public health. It can weaken the human immune system, cause discomfort, and reduce food production.
в о
s
p в
в в .s
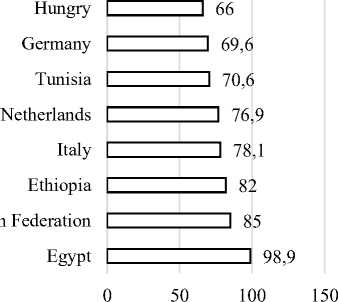
Prevalence of Sarcocystis infection in % age
Figure 3. Prevalence of Cattle Sarcocystis infection in selected African and European countries
Y Sarcocystis roimiicli(KY 120292.1)
^Sarcocystis romrneli (KYI20289.1)
•Snrcocyslis rcmmielKKY 120287 I)
° Sarcocystis bovite lis(KT900969.1)
( eSarcocyatis lxwifelia(KT900967.1)
*Sarcocystis bovifehs(KC2O9692.1) OSarcocyslis bovilclistK 1900961 I) a Sarcocystis bovifelis(KC209695,1) a Sai eocystia bovirelia(MT796924. 1) ( I $Saicocy si is bovifclis(ML 796911.1)
* Sarcocystis bovifelis(MT796906.1) L-®Snnx>cyiitis boviMia(KC20W>04 I)
1 Sarcocystis iioiiums(OQl90467.1)
'Sarcocystis homints(OQ 190167.1)
0.03
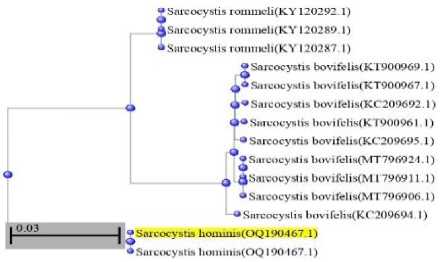
Figure 4. Phylogenetic tree for Sarcocystis hominis identification
This tree was constructed based on 13 sequences producing significant alignments.
Conclusion
According to the information reported in the literature, the viability of zoonotic sarcocysts remains intact when subjected to temperatures ranging from 0 to 4°C [6]. The Rwanda Inspectorate, Competition and Consumer Protection Authority (RICA), which is the nation's primary consumer rights watchdog, advises maintaining frozen temperatures between 2 and 4°C. According to the literature, the rule may reduce the risk of zoonotic diseases other than intestinal sarcocystosis. Therefore, more specific research on zoonotic Sarcocystis species in cattle and swine is recommended.
The Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) would not overlook human intestinal sarcocystosis, as its impact is severe on vulnerable individuals. Therefore, more studies are needed to gain insight into the parasite and develop more sophisticated systems to prevent and control the spread of the infection. Though foodborne illness from meat prevention and control are taken seriously in African countries, zoonotic sarcocystis infection is neglected, and there is limited research and reporting on this parasite in these countries. Therefore, more specific research on zoonotic sarcocysts is recommended.
Molecular methods are essential for detecting and diagnosing zoonotic Sarcocystis species. Due to cross-species transmission, the disease may decrease food availability in the food production system. It can cause abortion in cattle and pigs, stunted growth, weight loss, condemnation of infected meat tissue upon slaughter, and even death. Molecular and microscopic research methods should be implemented in meat processing plants and slaughterhouses.
Aknowledgement
I acknowledge the tremendous support from my co-author and my supervisor, Prof. Igor Glamazdin, for his advice and clear ideas for this work.
Список литературы Foodborne illnesses caused by tissue parasitic protozoa
- Amairia S., Amdouni Y., Rjeibi M.R., Rouatbi M. et al. First molecular detection and characterization of Sarcocystis species in slaughtered cattle in North-West Tunisia. Meat Science. 2016. vol. 122. pp. 55-59. https://doi.org/10.1016/j.meatsci.2016.07.021
- Ayazian Mavi S., Teimouri A., Mohebali M., Sharifi Yazdi M.K. et al. Sarcocystis infection in beef and industrial raw beef burgers from butcheries and retail stores: A molecular microscopic study. Heliyon. 2020. vol. 6. pp. e04171. https://doi.org/10.1016/j.heliyon.2020.e04171
- Dubey J.P., van Wilpe E., Calero-Bernal R., Verma S.K. et al. Sarcocystis heydorni, n. sp. (Apicomplexa: Sarcocystidae) with cattle (Bos taurus) and human (Homo sapiens) cycle. Parasitol Res. 2015. vol. 114. pp. 4143-4147. https://doi.org/10.1007/s00436-015-4645-2
- Fayer R., Esposito D.H., Dubey J.P. Human Infections with Sarcocystis Species. Clin Microbiol Rev. 2015. vol. 28. pp. 295-311. https://doi.org/10.1128/CMR.00113-14
- Hoeve-Bakker B.J.A., van der Giessen J.W.B., Franssen F.F.J. Molecular identification targeting cox1 and 18S genes confirms the high prevalence of Sarcocystis spp. in cattle in the Netherlands. International Journal for Parasitology. 2019. vol. 49. pp. 859-866. https://doi.org/10.1016/j.ijpara.2019.05.008
- Honda M., Sawaya M., Taira K., Yamazaki A. et al. Effects of temperature, pH and curing on the viability of Sarcocystis, a Japanese sika deer (Cervus Nippon centralis) parasite, and the inactivation of their diarrheal toxin. The Journal of Veterinary Medical Science. 2018. vol. 80. pp. 1337-1344. https://doi.org/10.1292/jvms.18-0123
- Hornok S., Mester A., Takács N., Baska F. et al. Sarcocystis-infection of cattle in Hungary. Parasites & vectors. 2015. vol. 8. no. 1. pp. 1-6. https://doi.org/10.1186/s13071-015-0685-9
- Januskevicius V., Januskeviciene G., Prakas P., Butkauskas D. et al. Prevalence and intensity of Sarcocystis spp. infection in animals slaughtered for food in Lithuania. Veterinarni Medicina. 2019. vol. 64. pp. 149-157. https://doi.org/10.17221/151/2017-VETMED
- Khalifa R.M.A., El-Nadi N.A.E.-F.A., Sayed F.G., Omran E.K. Comparative morphological studies on three Sarcocystis species in Sohag, Egypt. J Egypt Soc Parasitol. 2008. vol. 38. pp. 599-608.
- Lindsay D.S., Dubey J.P. Neosporosis, Toxoplasmosis, and Sarcocystosis in Ruminants. Veterinary Clinics of North America: Food Animal Practice. 2020. vol. 36. pp. 205-222. https://doi.org/10.1016/j.cvfa.2019.11.004
- Metwally A.M., Abd Ellah M.R., AL-Hosary A.A., Omar M.A. Microscopical and serological studies on Sarcocystis infection with first report of S. cruzi in buffaloes (Bubalus bubalis) in Assiut, Egypt. J Parasit Dis. 2014. vol. 38. pp. 378-382. https://doi.org/10.1007/s12639-013-0257-x
- Moré G., Pantchev A., Skuballa J., Langenmayer M.C. et al. Sarcocystis sinensis is the most prevalent thick-walled Sarcocystis species in beef on sale for consumers in Germany. Parasitol Res. 2014. vol. 113. pp. 2223-2230. https://doi.org/10.1007/s00436-014-3877-x
- Ortega Y.R., Sulaiman I.M. Protozoa: Sarcocystis, in: Encyclopedia of Food Safety. Elsevier, 2014. pp. 49-53. https://doi.org/10.1016/B978-0-12-378612-8.00137-2
- Rosenthal B.M. Zoonotic Sarcocystis. Research in Veterinary Science. 2021. vol. 136. pp. 151-157. https://doi.org/10.1016/j.rvsc.2021.02.008
- Rubiola, S., Civera, T., Panebianco, F., Vercellino, D., Chiesa, F., 2021a. Molecular detection of cattle Sarcocystis spp. in North-West Italy highlights their association with bovine eosinophilic myositis. Parasites Vectors 14, 223. https://doi.org/10.1186/s13071-021-04722-5
- Tumusiime M., Ntampaka P., Niragire F., Sindikubwabo T. et al. Prevalence of Swine Gastrointestinal Parasites in Nyagatare District, Rwanda. Journal of Parasitology Research. 2020. pp. 1-7. https://doi.org/10.1155/2020/8814136
- Vangeel L., Houf K., Chiers K., Vercruysse J. et al. Molecular-Based Identification of Sarcocystis hominis in Belgian Minced Beef. Journal of Food Protection. 2007. vol. 70. pp. 1523-1526. https://doi.org/10.4315/0362-028X-70.6.1523
- Vercruysse J., Fransen J., Goubergen M. The Prevalence and Identity of Sarcocystis Cysts in Cattle in Belgium. Journal of Veterinary Medicine, Series B. 1989. vol. 36. pp. 148-153. https://doi.org/10.1111/j.1439-0450.1989.tb00582.x
- Waine K., Bartley P.M., Cox A., Newsome R. et al. Molecular detection of Sarcocystis cruzi in three beef carcases with eosinophilic myositis lesions and in unaffected beef from animals in the same herd. Veterinary Parasitology: Regional Studies and Reports. 2022. vol. 33. pp. 100751. https://doi.org/10.1016/j.vprsr.2022.100751
- Woldemeskel M., Gebreab F. Prevalence of Sarcocysts in Livestock of Northwest Ethiopia. Journal of Veterinary Medicine, Series B. 1996. vol. 43. pp. 55-58. https://doi.org/10.1111/j.1439-0450.1996.tb00287.x


