Iron deficiency and milk fortification
Автор: Mussayeva S.J., Iskakova Zh.A., Aitbaeva A., Dautkanova D.R., Iztileuov M.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Технология пищевой и перерабатывающей промышленности
Статья в выпуске: 1 (147), 2025 года.
Бесплатный доступ
Modern food products do not contain a high content of nutrients, in particular minerals. The consumption of such products affects not only the health of the nation but also the economy of the country. According to the World Health Organization, the lack of the mineral element iron remains relevant, especially among children and pregnant women not only in developed countries, but all over the world. This article presents studies of the effect of cow's milk enrichment on the physico-chemical and organoleptic qualities of fortified milk. The cow's milk with a fat content of 3.6 and 2.8% was enriched with iron sulfate (FeSO4.7H2O) and trivalent ammonium citrate (C6H8FeNO7). The samples of Ferrous Sulfate (10mg) and Ferric Ammonium Citrate (10mg) showed significant changes in milk with fat content 2.8 % fortification. The effect of iron enrichment on the process of milk fermentation was also studied, and a milk sample was taken. The milk sample fortified with iron had a considerable acidity increase on 3 h from 22°T to 800T, this is by 37 % compared to the control sample. The effect of iron enrichment on the fermentation process or the supporting role of iron in the growth of fermenting bacteria is still in question, although a number of studies have been conducted in this area, but more clarity and specific research and experiments on this issue are still needed.
Micronutrient, iron deficiency, food enrichment, milk, anemia, pasteurized milk, physicochemical properties
Короткий адрес: https://sciup.org/140310056
IDR: 140310056 | DOI: 10.48184/2304-568X-2025-1-11-19
Текст научной статьи Iron deficiency and milk fortification
2024;1(2(14)):66-73.
In recent years, due to urbanization, the quality of the population's diet has deteriorated sharply. The use of fast food and refined products all affects the health of the nation. The lack of quality food from time to time is unlikely to cause serious health problems, but the constant consumption of such products, especially daily products such as bread and dairy products, can negatively affect human health. The human body needs to get the necessary nutrients from food: proteins, fats, vitamins and minerals in the right quantities so that it can function optimally. The consumer's goal is to choose a variety of foods with high nutritional content in most cases.
Iron deficiency is the most common nutritional deficiency in the world. Apart from the fact that it affects a large number of children and women in developing countries, it is the only micronutrient deficiency that also significantly exacerbates the situation.
The statistics are stunning: 2 billion people, which is already over 30% of the world’s population, are anemic, many due to iron deficiency. This disease reduces the ability of individuals and entire groups of the population to work, which leads to serious economic consequences and hinders national development. According to statistics, it is the most vulnerable, the poorest and the least educated who are disproportionately affected by iron deficiency [1-3].
According to WHO data on human mortality, about 0.8 million deaths (1.5% of the total) may be caused by related to iron deficiency every year. Also in terms of the loss of healthy life, which is technically expressed in disability-adjusted life years, iron-deficiency anemia results in 25 million years lost [1-4].
Based on joint research studies by WHO and UNICEF, the central Asian republics and Kazakhstan had an anemia prevalence rate of more than 50% among women and young children and based on dietary studies it was found out that the majority of all anemia in this region was caused by iron deficiency. More than a decade later, despite of economic and lifestyle improvements anemia in Kazakhstan is in a moderate condition according to WHO data and other studies done in this country.
There are concerns about biophysico-chemical modifications, organoleptic changes, iron bioavailability and tissue distribution as a result of such an intervention. In fact, all these changes depend on various parameters and factors, in particular, the physico-chemical properties of the added iron (iron valence, solubility, degree of chelation, complexation), the physico-chemical properties of iron can increase or decrease iron absorption and physiological state [4-8].
Today, the fortification of dairy products with vitamins and minerals is also practiced all over the world. In the USA and France, dairy products are enriched with vitamin D, as a result, there is a noticeable decrease in the incidence of rickets, largely due to this practice. In Kazakhstan, the dairy company "Food Master" (France) produces ultra-pasteurized cow's milk and it is also enriched with vitamin D. In practice, to obtain a better organoleptic result, whole cow's milk is usually enriched with various iron additives, iron sulfate, trivalent ammonium citrate and their encapsulated forms or chelated forms such as Naphtha and iron bisglycinate are used [9-12].
Materials and research methods
Analyzing physicochemical properties of the milk
Milk quality was determined using a generally accepted technique based on Ekomilk Ultra. Ekomilk Ultra is an automatic device capable of determining fat, solids nonfat (SNF), density, protein, freezing point, added water and pH of the milk sample. In setting menu there are options to analyze cow or goat milk and pasteurized and non-pasteurized milk samples. In our case the required setting was cow milk 2 for pasteurized milk. The milk sample shall be poured into special vials which will fit onto the device’s testing probe. Upon running the analysis, it will take a few seconds to show the results which can be printed out using the attached printing device.
Choosing the right iron fortificant
In order to choose the right form of iron fortificant, we should also consider the local and or regional food additives’ regulations which introduce the permitted forms of different food additives inclusive of minerals and vitamins to be used in food industry. In Kazakhstan the standard food additives list introduces a number of iron fortificants permitted to be added to food products which include ferrous gluconate, ferrous sulfate, ferrous lactate, ferrous fumarate, ferric diphosphate (pyrophosphate), ferrous citrate, ferric ammonium citrate, ferric bisglycinate and NaFeEDTA [13]. However, there are special recommendations for fortifying fluid milk with iron and they include specific forms of iron fortificants considering the bioavailability and sensory effects of them. For example, the recommended iron forms to use as fortificant in fluid milk by FAO/WHO are ferric ammonium citrate (C6H8FeNO7: iron content 21%), ferrous bisglycinate (C4H8FeN2O4: iron content 27%) and micronized ferric pyrophosphate (Fe4(P2O7)3: iron content 30%). As these forms are proven to cause less-to-no sensory impacts on the fluid milk.
Calculating the right fortificant amount
Another important point is that every iron fortificant form has a different iron content based on its chemical formula and accordingly to calculate the appropriate amount of iron fortificant which will be added we should consider the iron content of a selected iron fortificant. For example, ferrous sulfate with the chemical formula FeSO 4 .7H 2 O has a total molecular mass of 278.01 of which 20% is iron, hence, if we are going to add 5mg of iron in the milk we should weight 25g of ferrous sulfate to contain this amount of iron.
Equation summary →
100mg FeSO4 20mg Fe =>
500/20=25mg FeSO4
X 5mg Fe
After finishing the analysis, the milk properties were shown as the following:
Table 1. Initial physicochemical analysis of the pasteurized cow milk sample
|
Fat % |
SNF % |
Density kg/m3 |
Protein % |
Freezing point °C |
pH |
Added Water % |
|
3.60 |
8.86 |
29.70* |
3.34 |
58.10 |
12.87R |
0.00 |
* the unit figure is abbreviated from 1029.70 (the range is 1020-1040)
Results and discussion
Iron fortificant addition.
Two milk samples with fat percentages of 3.2 (Control 1) and 2.5 (Control 2) from a local dairy company “Мое” were purchased. Before determining the sample’s physicochemical properties, it was left at room temperature for half an hour and then using the “Ecomilk” instrument its physicochemical properties including fat, SNF, protein, pH and density were determined. Then the main part of the experiment i.e. adding iron fortificant salts started.
The next step was addition of iron fortificants to the sample. The following iron fortificants in the form of iron salts were purchased from local companies “Veld” and “Labhimprom” (Figure 1).

Figure 1. Iron salts used for fortification experiment
Ferrous Sulfate (FeSO 4 .7H 2 O) and Ferric Ammonium Citrate: (C 6 H 8 FeNO 7 ).
According to the fortificants’ formulas the percentage of iron in each fortificant was calculated as the following:
Ferrous Sulfate (FeSO4.7H2O)→ Total molar mass: ~278 – iron percentage: ~20%
Ferric Ammonium Citrate (C 6 H 8 FeNO 7 ) → Total molar mass: ~262 – iron percentage: ~20%
The daily iron intake (DRI) for iron in Kazakhstan is 18-20 mg for women and 10-12 mg for men [15], accordingly the maximum amount possible i.e. 20 mg was considered to be added to the sample. The required amounts of fortificants to be containing the target DRI were calculated based on the previous section “Calculating the right fortificant amount”. Therefore, based on the fortificants’ formulas we needed to weight 100 mg of ferrous sulfate and ~100mg of ferric ammonium citrate. Using the laboratory sensitive scale, the required amounts of the fortificants were precisely weighed on filter papers and poured into separate 250 ml beakers. Then 200 ml of milk was added to each beaker. The beakers were put on the disk lab with a magnet inside of each in order to thoroughly mix the iron fortificants with the milk.
The physicochemical properties after iron fortification
After fortification process, the samples were analyzed for any possible physicochemical changes using the “Eсomilk” instrument. The results were almost the same while there were some slight increases in the amounts of SNF, density and freezing point for Ferric Ammonium Citrate-fortified sample. The test results after the fortification with 20 mg and 10 mg of iron salts are shown in Table 2.
Table 2. Physicochemical analysis of pasteurized cow milk after iron fortification
|
Iron salt added |
Fat, % |
SNF, % |
Density, kg/m3 |
Protein, % |
Freezing point, °C |
pH |
|
Control 1 |
3.60 |
8.86 |
29.70 * |
3.34 |
58.10 |
12.87R |
|
Simple 1 Ferrous Sulfate (20 mg) |
3.58 |
8.73 |
29.20 * |
3.29 |
57.30 |
12.90R |
|
Simple 2 Ferric Ammonium Citrate (20 mg) |
3.59 |
9.23 |
31.00 * |
3.48 |
60.40 |
12.90R |
|
Control 2 |
2.80 |
10.60 |
35.70* |
4.01 |
67.80 |
12.50R |
|
Simple 3 Ferrous Sulfate (10 mg) |
2.78 |
8.44 |
28.70* |
3.18 |
55.60 |
12.60R |
|
Simple 4 Ferric Ammonium Citrate (10 mg) |
2.79 |
8.46 |
28.80* |
3.19 |
55.70 |
12.59R |
* the unit figures are abbreviated from the range 1020-1040
The results of the study showed that the fat content in all samples decreased slightly by 0.50.4% compared to the control samples. SNF increased by 4.1% in the 2nd sample compared with the 1st control, and decreased in the 3.4 samples by 18-20%, respectively, compared with the 2nd control.
The density index changed slightly, except for 3.4 samples, which decreased by 19% compared to the 2nd control.
The protein in samples 3 and 4 decreased by 20% compared to the same Control -2.
The addition of iron also affected the freezing point of milk, noticeable observations were shown in samples 3 and 4, which showed 17.9-17.7%, respectively, compared with control 2.
The samples of Ferrous Sulfate (10 mg) and Ferric Ammonium Citrate (10 mg) showed significant changes in milk with fat content 2.8 % fortification.
Organoleptic changes
The fortified samples were then tested for organoleptic changes in intervals of immediately after the iron addition, one hour later, after boiling and 24 hours later after being stored in the fridge. The reference regulation used for sensory properties is Kazakhstan state standard for milk organoleptic evaluation known as GOST [13].
Color. It is noteworthy that immediately after the addition of milk onto iron fortificant salts the color of the milk changed according to the color of the salts. Ferrous Sulfate changed the milk color to a darker tone or a greenish metallic color and Ferric Ammonium Citrate changed it to a pale brown color. The color change was still persistent after the mixing process and it stayed the same one hour later. After boiling the samples their colors were still the same with a darker tone as a result of boiling process. No more color changes were visible 24 hours later after the samples were stored in the fridge. The following figures - 2 show the color changes in intervals of immediately after fortificant addition, after one hour, after boiling and 24 hours later in contrast with the control milk sample.
Odor. The sample to which Ferric Ammonium Citrate was added didn’t show a specific odor change immediately after addition to iron salts while the sample with Ferrous Sulfate represented a slightly barn odor. The odor change was the same after one hour but the barn odor of Ferrous Sulfate sample was decreased after boiling and it remained the same for both samples after 24 hours while they were stored in the fridge overnight.
Taste. The sample to which Ferric Ammonium Citrate was added didn’t show a specific taste change immediately after addition to iron salts while the sample with added Ferrous Sulfate tasted slightly metallic. The flavor status was the same after one hour but it was decreased for Ferrous Sulfate sample after boiling and it remained the same for Ferric Ammonium Citrate-fortified sample after 24 hours in the fridge while the metallic taste of Ferrous Sulfate-fortified sample seemed already gone.
Flavoring: Masking the unpleasant sensory effects of iron fortificants [14,15].
Flavoring agents are the largest single group of food additives. Food and beverage applications of flavors include dairy, fruit, nut, seafood, spice blends, vegetables and wine flavoring agents. They may complement, magnify, or modify the taste and aroma of the foods. In order to mask the unpleasant sensory effects caused by ferrous sulfate fortification, we added two drops of butter vanilla flavor (Givaudan company) which showed to be effective and helped to improve the unpleasant sensory changes to a satisfactory level. Adding butter vanilla flavor completely masked the unpleasant odor and flavor changes caused by ferrous sulfate and upon the sensory tests the flavored fortified sample was received satisfactorily and pleasant. To mask the light brown color or creamy tone caused by Ferric Ammonium Citrate, the use of cocoa flavoring had a positive effect on sensory tests.
The effect of iron fortification on milk fermentation
Fermented milk, such as yogurt, is consumed as a dessert, drink, or snack due to its characteristic features: a pleasant aromatic flavor, thick creamy consistency, and reputation as a food product associated with health benefits [16, 17]. To obtain such products, the effect of fortified milk on the milk fermentation process was further investigated.
To study the effect of iron fortification on the milk fermentation process, a milk sample containing 2.5% fat was selected and fortified with ferric ammonium citrate. A bacterial fermentation culture was then added to evaluate the fermentation process quality in the presence of the iron salt.
For the experiment, 1000 ml of the milk sample was mixed with the bacterial fermentation culture extract. Half of the mixture (500 ml) was further mixed with ferric ammonium citrate as an iron fortificant, while the remaining 500 ml was left as a control. The iron-fortified milk sample was then divided into five 100 ml beakers, labeled Fe-BC, and numbered 1 to 5 (Fe-BC 1, 2, 3, 4, 5) (Figure 2).

Figure 2. The DRI for iron in Kazakhstan is 20 mg for women
The control milk sample which contained only bacterial fermentation culture was also divided in 5 beakers of 100 ml capacity labelled as
BC and numbered 1 to 5 (BC 1, 2, 3, 4, 5). The samples were all put in the incubator with a temperature set to 42°C to let the fermentation process begin. Two milk samples, one from Fe-BC group and one from BC group were taken out every one hour to analyze their acidity degree using the titration method (titratable acidity) which is expressed in Termer degrees. While taking out a pair of samples the other samples in the incubator were shaken to let the fermentation process take place optimally. The following Figure 3 shows the acidity change during the 5-hour period of fermentation.
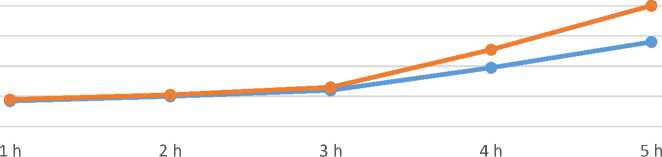
Time (hour)
BC Fe-BC
Figure 3. Fermentation process for cultured milk fortified with Ferric Ammonium Citrate (Fe-BC) versus cultured milk (BC) control
Fermentation activity was observed in both cases— in the presence of Ferric Ammonium Citrate (Fe-BC) and in the iron-free control sample (BC)—after 3 hours from the start of the milk fermentation process. Notably, the iron-fortified milk sample showed a significant increase in acidity within 2 hours, rising from 22 °T to 80 °T, which is 37% higher compared to the control sample.
The effect of iron fortification on the fermentation process, particularly its potential role in supporting the growth of fermenting bacteria, remains an open question. Although several studies have been conducted in this area, the results are not entirely conclusive. Therefore, further research and more specific experiments are needed to provide greater clarity on this topic.
We also tried the same experiment with ferrous sulfate salt to evaluate the fermentation process in a different medium, the results of the study are shown in Figure 4.
60 о
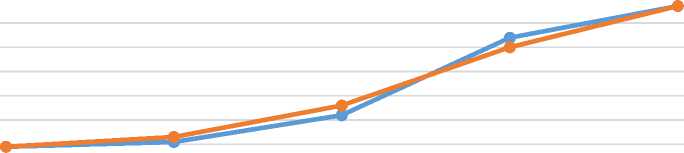
1 h 2 h 3 h 4 h 5 h
Time (hour)
BC Fe-BC
Figure 4. Fermentation process for cultured milk fortified with Ferrous sulfate (Fe-BC) versus cultured milk (BC) control
It was observed that both fermentation processes in this experiment progressed similarly, reaching 77°T after 5 hours of incubation. After this point, the control and iron-fortified samples appeared nearly identical in terms of thickness and overall appearance.
It is noteworthy that the milk sample fortified with iron showed much more thickness and viscosity compared to the control sample which is yet another sign of more fermentation that took place in it. Recent studies suggest that the presence of iron can affect the growth of lactic bacteria to some extent.
Conclusions
Food fortification remains a valuable method for delivering iron to populations that constitutes a significant proportion of their food. However, technical problems may limit the quantity of bioavailable iron that can be delivered. Further research is needed to establish improved methods for ensuring that adequate quantities of bioavailable iron are supplied. It is noteworthy that immediately after adding iron-enriching salts to the milk, the organoleptic parameters of the milk changed depending on the color of the salts. The sample to which ammonium trivalent citrate was added showed no change in the specific odor immediately after the addition of iron salts, while the sample to which iron sulfate was added had a faint odor of barn. The effect of iron fortification on the fermentation process or the assisting role of iron in the growth of fermenting bacteria is still a question although there have been a number of studies done in this area. However, since the results are not entirely absolute and determining, there is still need for more clarity and specific research and experiments on this topic.
Finally, in case of using milk as the fortification vehicle, the issue of intolerance to milk components would be of importance. It should be evaluated, through sampling and estimation studies within the society, how many people do not have issues with drinking milk. Such issues include, but are not limited to, milk allergy, intolerance to milk proteins, lactose intolerance, and galactosemia. Though, multiple milk iron fortification programs have been performed around the world and resulted in promising health changes within the target populations.


