Methodology for obtaining complex bioactive substences from plant resources
Автор: Merenkova S.P., Yan Huang, Wu Siyu, Chzhou Khenkhen
Рубрика: Биохимический и пищевой инжиниринг
Статья в выпуске: 3 т.11, 2023 года.
Бесплатный доступ
Natural biologically active substances obtained from various plant sources are highly valued for their low toxicity, wide range of sources and high yield of final components. It has been established that biologically active (antioxidant) properties are characterized by such biochemical substances as flavonoids, polyphenols, vitamins, especially carotenoids, and active polysaccharides. Rosemary contains significant concentrations of biologically active components, the most important of which are essential oils, carnosol, carnosic acid and rosemary acid. As a rule, there is a synergistic effect between different types of antioxidants. The antioxidant capacity of bioactive components after mixing can be significantly higher than that of the original antioxidants, and it is more effective, less energy-consuming, safer and less toxic. Rosemary leaf extracts can enhance their antioxidant activity when interacting with natural carotenoids. The purpose of this study was to design and analyze the optimized parameters for obtaining a complex of biologically active substances based on the principles of synergism of carrot vitamins and rosemary polyphenolic compounds. To confirm and comparatively analyze the bioactive properties of the obtained complex extracts, studies of antiradical activity, content of polyphenolic and flavonoid compounds were performed. The monoextracts based on 70 % ethanol as well as distilled water extract of rosemary were characterized by the highest DPPH activity and significant concentration of polyphenolic substances. The efficiency of complex plant ethanol extracts was established, the highest concentration of bioactive components and antioxidant activity in the range of 86.9-92.5 % was observed. As a result of this study, the feasibility of application of different extraction agents for the isolation of bioactive compounds from rosemary and carrots was scientifically substantiated, and bioactive components with proven antioxidant properties were developed.
Bioactive components, polyphenols, antioxidant properties, rosemary leaves, extraction, synergistic effect
Короткий адрес: https://sciup.org/147241713
IDR: 147241713 | УДК: 581.192+543.48 | DOI: 10.14529/food230310
Текст научной статьи Methodology for obtaining complex bioactive substences from plant resources
Natural bioactive compounds that are derived from various plants are highly praised for their low toxicity, wide range of sources, and huge yields. It has been established that biologically active (antioxidant) properties are characterized by such biochemical substances as flavonoids, polyphenols, vitamins, specially carotenoids, and active polysaccharides [1]. Different natural antioxidants have different antioxidant mechanisms, so there are different interactions among them, including additive, antagonistic and synergistic effects. Among them, the synergistic effect has attracted the most attention, because it can produce a variety of compound antioxidant. The mechanism of synergistic effects includes several types. There are many related studies on the synergy between polyphenols and vitamins and the synergy between flavonoids and polysaccharides [2].
Plant polyphenols refer to the general group of polyhydric phenolic compounds that widely exist in plants. They are secondary metabolites synthesized by plants during normal development or under stress conditions, In their structure, there is at least one aromatic ring with one or more hydroxyl groups. Polyphenolic compounds react
with excess free radicals in the human body to generate relatively more stable phenolic oxygen free radicals, which can protect the human body from damage [3]. Currently, more than 8,000 polyphenols have been isolated and identified. In recent years, the active function of polyphenolic compounds has gradually attracted researcher's attention. With the discovery of biologically active functions such as slowing down the aging, preventing cardiovascular and cerebrovascular diseases, eliminating free radicals in the body, counteracting lipid oxidation, anti-radiation and anti-cancer protection, – the research and application of polyphenols have been paid more and more attention.
Rosemary is an evergreen, winter-hardy plant that can be grown in Mediterranean climates and often appears on people's tables as a spice. As a natural preservative and natural antioxidant, rosemary has played an important role in food preservation since ancient times. Rosemary contains significant concentrations of biologically active components, the most important of which are essential oils, carnosol, carnosic acid and rosmarinic acid [4]. Rosemary has been shown to be pharmacologically active and contains components that are potential for medical
applications. Thus bioactive components of rosemary inhibit the development of malignant cells in tissues, melanoma cells and leukemia cells. Based on bioinformatics, rosemary can be used for biological targeting therapy of cancer, and the different molecular targets regulated by the active components of rosemary are useful indicators of success in clinical trials of cancer chemoprevention. The table 1 lists active ingredients of rosemary, their applications and ad-
vantages. Figure 1 shows the molecular structure of rosemary BAS.
There is generally a synergistic effect between the different types of antioxidants. According to a certain proportion, the antioxidant capacity of the bioactive substances after compounding can be significantly increased, and become more efficient, less energy-consuming, and less toxic. At present, researchers study the antioxidant synergy between vitamins and polyphenols; flavo-
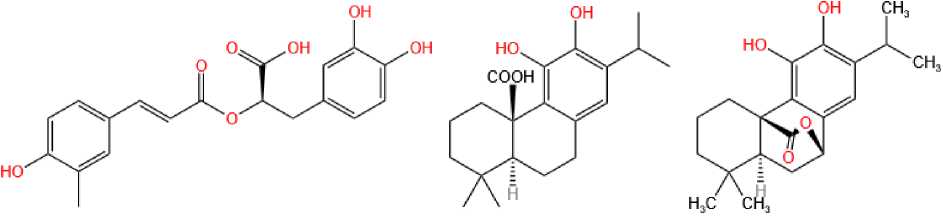
Rosmarinic acid (RA)
Carnosic acid(CA)
Carnosol(CN)
Fig. 1. Main bioactive substances of rosemary
Table 1
List of active ingredients of rosemary and their applications and advantages
|
Bioactive compounds |
Direction of application |
Beneficial effect |
Reference |
|
Carnosic acid, rosemic acid and carnosol |
Antioxidant |
Safe and efficient free radical scavengers can effectively prevent the oxidation and rancidity of oil and prevent the fading or discoloration caused by oxidation |
[5] |
|
2-pinene, camphor and 1,8-eucalypt hormone |
Antimicrobial |
Can inhibit the growth and survival of insects and eggs, trace and efficient |
[6] |
|
Carnosic acid caffeic acid, and Luteolin-7-O-glucuronide |
Anti-depression |
By regulating catecholamines, it can also reduce the toxic damage of corticosterone and protect nerve cells |
[7] |
|
Carnosic acid , carnosol |
Regulation of metabolism |
Rosemary extract improved plasma total cholesterol, LDL, and triglycerides in mice fed on a high-cholesterol diet, as well as decreased fasting plasma glucose, plasma total cholesterol, and free fatty acids |
[8] |
|
Luteolin, carnosic acid and rosmarinci acid |
Anti-nerve injury |
Can reduce the expression of Hsp 47 in nerve cells, thereby improving the damage degeneration of nerve cells in a stressful environment |
[9] |
|
Carnosic acid |
Antiinflammatory |
Lower the factors involved in inducing the development of inflammation, such as myeloperoxidase, interleukin 1- β, tumor necrosis factor- α, and reduced leukocytes |
[10] |
|
Carnosic ac-id 、 carnosol salviol |
Antineoplastic |
Tumor cell survival is affected by promoting cell apoptosis and inhibiting the phosphatidylinositol-3-kinase / protein kinase (PI3K / Akt) pathway |
[11] |
Table 2
Extraction methods for obtaining plant bioactive substances
|
Extraction Method |
Extraction principle |
Benefit |
Reference |
|
Maceration |
Conducted by soaking plant materials in solvent at room temperature to obtain plant extract. |
Simple operation and low facility requirements |
[14] |
|
Percolation |
Conducted by passing solvent through plant materials at controlled rate |
Simple operation, continuous operation |
[ %] |
|
Decoction |
Boiling plant materials in water to obtain plant extracts. |
Good separation effect and easy operation |
[16] |
|
Heat reflux extraction and Soxhlet extraction |
Utilize reflux technique but with different apparatus to extract active compounds from plant materials. |
The extract has high purity and is easy to operate |
[17] |
|
Ultrasound-assisted extraction |
Utilizes cavitation caused by ultrasound effects in solvent to enhance the Extraction of active compounds from plant materials |
Higher yields, shorter extraction times, less energy and solvent consumption |
[18] |
|
Supercritical fluid extraction |
Supercritical fluid has strong penetrating power, high density and good solubility |
Does not destroy active ingredients, no organic solvent residue |
[19] |
|
Microwave-assisted extraction |
Microwave energy is absorbed by polar substances inside the cell, generating a lot of heat, which creates holes and cracks in the cell wall |
Wide adaptability, low temperature, high extraction efficiency |
[20] |
|
Pressurized liquid extraction |
In pressurized liquid extraction, the solvent is heated above its atmospheric boiling point and maintained at high pressure down so that they remain liquid. Use temperatures and pressures below the critical point of the solvent |
Can dissolve more active substances, replacing organic solvents with water |
[21] |
|
Enzyme-assisted extraction |
Some enzymes are able to hydrolyze plants cell wall to promote the release of active compounds from plant matrixes |
Can be used to enhance the extraction of active compounds from plant material |
[22] |
|
High-voltage-assisted extraction |
When pulses of high voltage at very short time are applied to cells, lipid bilayers of cell membrane are disrupted, forming temporary pores that significantly increase mass transfer between inside and outside of the cells |
The ability to extract active compounds from plant material can be improved |
[23] |
Table 2 (End)
|
Extraction Method |
Extraction principle |
Benefit |
Reference |
|
Ionic liquids or deep eutectic solvents-based extraction |
Solvent properties significantly affect the extraction of active compounds. Therefore, if the solvent properties can be tailored, will facilitate extraction |
Solvent properties can be tailored which will facilitate extraction |
[24] |
|
Adsorption |
Adsorption technique makes use of solid adsorbents to adsorb targeted active compounds in crude extracts to achieve the purpose of extraction and isolation |
Consumption of organic solvents, extraction time, costs, waste generated can be reduced, and no pretreatment is required |
[25] |
Table 3
Composition of plant extracts samples
|
Group cod |
Solution composition |
Proportion of extracts |
|
R-1 |
Rosemary Extract (distilled water) |
1.0 |
|
R-2 |
Rosemary Extract (ethanol) |
1.0 |
|
R-3 |
Rosemary Extract (petroleum ether) |
1.0 |
|
C-1 |
Carrot Extract (petroleum ether) |
1.0 |
|
C-2 |
Carrot Extract (ethanol) |
1.0 |
|
RC-1 ethanol |
Rosemary Extract (distilled water)+ Carrot Extract (ethanol) |
1 : 1 |
|
RC-1 petrol |
Rosemary Extract + Carrot Extract (Petroleum Ether) |
1 : 1 |
|
RC-3 ethanol |
Rosemary Extract + Carrot Extract (ethanol) |
1 : 1 |
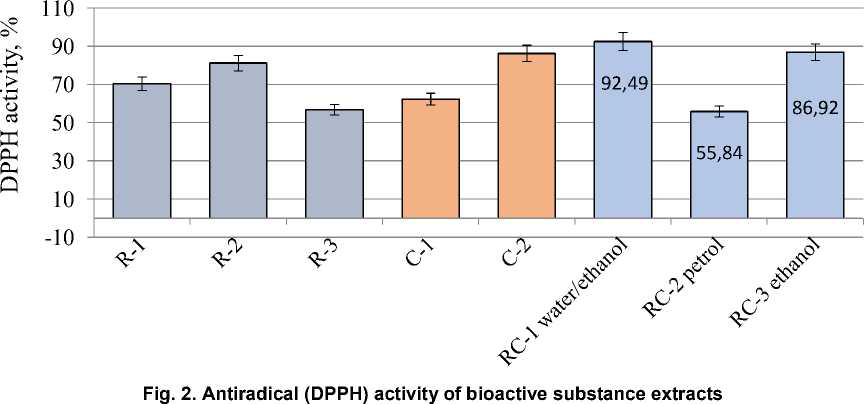
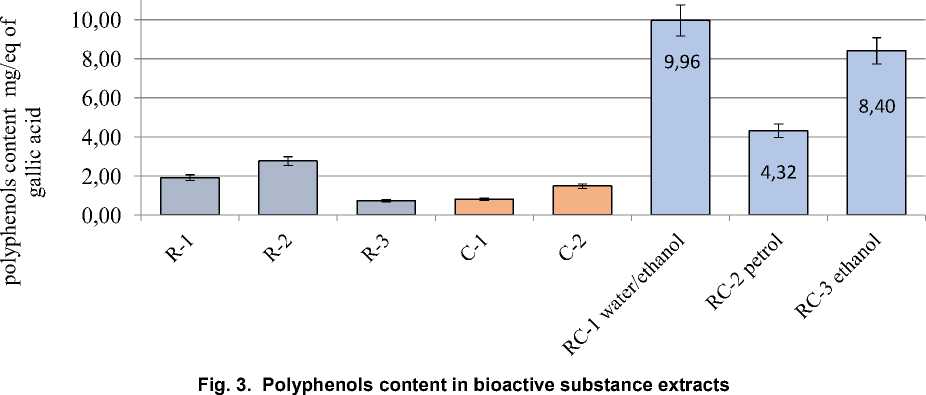
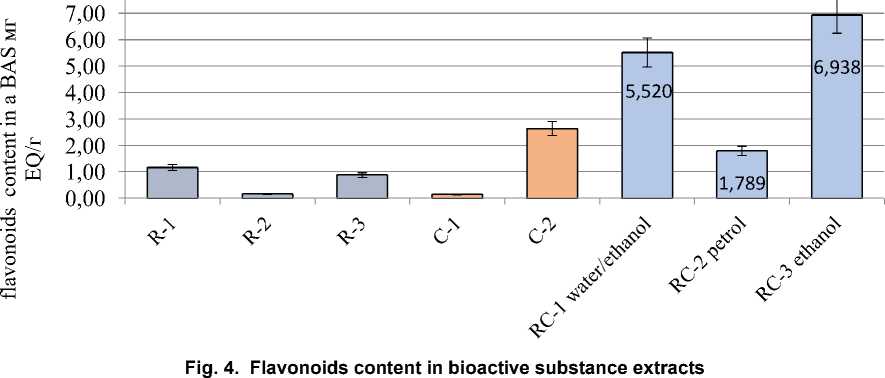
stances. The efficiency of complex plant extracts based on distilled water and ethanol was established, the highest concentration of bioactive components and antioxidant activity in the range of 86.9–92.5 % was observed.
Conclusion
Bioactive components, that have been extracted from natural plant sources are characterized by pronounced bioactive and antioxidant properties, which depend on the extraction method. The biological activity of the plant sub-
stances is depending on the content of polyphenolic and vitamin compounds, as well as their synergistic interaction. Rosemary leaf extracts can enhance their antioxidant activity when interacting with natural carotenoids. As a result of this study, the feasibility of application of different extraction agents for the isolation of bioactive compounds from rosemary and carrots was scientifically substantiated, and bioactive components with proven antioxidant properties were developed.
Список литературы Methodology for obtaining complex bioactive substences from plant resources
- Lee J., Song Y., Son H., Kim S., Lee K.H., Bazarragchaa B., Lee C., Yoo H.Y. Phytochemical and Antioxidant Characterization of Extracts from Unexplored Medicinal Plants Salix schwerinii and Salix kochiana. Horticulturae, 2023, vol. 9, no. 955. DOI: 10.3390/horticulturae9090955.
- Jing G. Natural antioxidants and their synergistic effects. Journal of Food Safety and Quality Inspection, 2020, vol. 11, no. 6, pp. 1859–1864.
- Polyphenols: antioxidants and beyond. The American Journal of Clinical Nutritio. Oxford Aca-demic [Electronic resource]. URL: https://academic.oup.com/ajcn/article/81/1/2 %S/ 4607494?login=true (accessed: 20.08.2023).
- Nieto G., Ros G., Castillo J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines, 2018, vol. 5(3), no. 98. DOI: 10.3390/medicines5030098
- Moreno S., Scheyer T., Romano C. S., Vojnov A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radical Research, 2006, vol. 40, no. 2. DOI: 10.1080/107 %760500473834.
- Jiang Y., Wu N., Fu Y., Wang W., Luo M. et al. Chemical composition and antimicrobial activ-ity of the essential oil of Rosemary. Environmental Toxicology and Pharmacology, 2011, vol. 32, no. 1, pp. 63–68.
- Wang H., Provan G.J., Helliwell K. Determination of rosmarinic acid and caffeic acid in aro-matic herbs by HPLC. Food Chemistry, 2004, vol. 87, no. 2, pp. 307–311.
- Hassani F.V., Shirani K., Hosseinzadeh H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn-Schmiedeberg’s Arch Pharmacol, 2016, vol. 389, no. 9, pp. 931–949.
- EL Omri A., Junkyu Han J., Hashizume R. Ron et al. Anti-Neuronal Stress Effect of Tunisian. Conference: Desert Technology IV. Journal of Arid land studies At: Tunisia. Volume: JALS 19 (1).
- Anti-Inflammatory Effects of Supercritical Carbon Dioxide Extract and Its Isolated Carnosic Acid from Rosmarinus officinalis Leaves. Journal of Agricultural and Food Chemistry [Electronic re-source]. URL: https://pubs.acs.org/doi/full/10.1021/jf104837w (accessed: 18.08.2023).
- Tai J. Cheung S., Wu M., Hasman D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine, 2012, vol. 19, no. 5, pp. 436–443.
- Olszowy-Tomczyk M.; Wianowska D. Antioxidant Properties of Selected Flavonoids in Binary Mixtures– Considerations on Myricetin, Kaempferol and Quercetin. International Journal of Molecular Sciences, 2023, vol. 24. 10070. DOI: 10.3390/ijms241210070.
- Jimenez-Escrig A.. Polyphenol and Carotenoid Protection in Biological Systems Through the Modulation of Antioxidant Enzymes. Current Enzyme Inhibition, 2006, vol. 2, pp. 231–248. DOI: 10.2174/ %7340806777934793.
- Ćujić N. Šavikin K., Janković T., Pljevljakušić D., Zdunić G., Ibrić S. Optimization of poly-phenols extraction from dried chokeberry using maceration as traditional technique. Food Chemistry, 2016, vol. 194, pp. 135–142.
- Pramparo M., Gregory S., Mattea M. Immersion vs. percolation in the extraction of oil from oleaginous seeds. Journal of the American Oil Chemists’ Society, 2002, vol. 79, no. 10, pp. 955–960.
- Kaneria M., Kanani B., Chanda S. Assessment of effect of hydroalcoholic and decoction methods on extraction of antioxidants from selected Indian medicinal plants. Asian Pacific Journal of Tropical Biomedicine, 2012, vol. 2, no. 3, pp. 195–202.
- Zhang M., Zeng, G., Pan, Y., Qi, N. Difference research of pectins extracted from tobacco waste by heat reflux extraction and microwave-assisted extraction. Biocatalysis and Agricultural Biotechnology, 2018, vol. %, pp. 359–363. DOI: 10.1016/j.bcab.2018.06.022
- Vilkhu K., Mawson R., Simons L., Bates D. Applications and opportunities for ultrasound as-sisted extraction in the food industry – A review. Innovative Food Science & Emerging Technologies, 2008, vol. 9, no. 2, pp. 161–169.
- Paulaitis M.E. Supercritical fluid extraction. Reviews in Chemical Engineering. Walter de Gruyter GmbH & Co. KG, 1983, vol. 1, no. 2, pp. 179–250.
- Sparr Eskilsson C., Björklund E. Analytical-scale microwave-assisted extraction. Journal of Chromatography A., 2000, vol. 902, no. 1, pp. 227–250.
- Mustafa A., Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Analytica Chimica Acta, 2011, vol. 703, no. 1, pp. 8–18.
- Puri M., Sharma D., Barrow C.J. Enzyme-assisted extraction of bioactives from plants. Trends in Biotechnology, 2012, vol. 30, no. 1, pp. 37–44.
- Chuo S.C. Nasir H.M., Siti Hamidah Mohd-Setapar H., Mohamed S.F. et al. A Glimpse into the Extraction Methods of Active Compounds from Plants. Critical Reviews in Analytical Chemistry, 2022, vol. 52, no. 4, pp. 667–696.
- Dai Y., van Spronsen J., Witkamp G.J., Verpoorte R., Choi Y.H.. Ionic Liquids and Deep Eu-tectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. Journal of Natural Products, 2013, vol. 76 (11), pp. 2162–2173. DOI: 10.1021/np400051w.
- Mohammed R.R. Inaam A.R. Ibrahim, Taha A.H., Gordon Mckay G. Waste lubricating oil treatment by extraction and adsorption. Chemical Engineering Journal, 2013, vol. 220, pp. 343–351.


