Monitoring the presence of residues of lasalocid in feed samples HPLC-MS/MS
Автор: Rosa E. gavilN., Carolina neboT., Alejandra iglesiaS., Beatriz vazqueZ., Jose M. mirandA., Carlos M. francO., Alberto cepedA.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Естественные науки
Статья в выпуске: 4 (100), 2013 года.
Бесплатный доступ
Coccidiostats are veterinary medicine licensed to prevent and treat coccidiosis and histomoniasis. Due to the unavoidable carryover of these substances during the fabrication the European Commission decided to set up maxim level of coccidiostats in non-target feeds (Regulation 574/2011), including lasalocid. In this work an easy and cheap HPLC-MS/MS method for the analysis of lasalocid in medicated and contaminated feed is presented. The method only required 5 ml of acetonitrile and the detection is achieved for concentration between 60 and 1500 μg/Kg. The method full fills the criteria of the European Decision 2002/657/EC for the detection of lasalocid.
Feeds, lasalocid, hplc-ms/ms method
Короткий адрес: https://sciup.org/140205011
IDR: 140205011 | УДК: 636.085
Текст научной статьи Monitoring the presence of residues of lasalocid in feed samples HPLC-MS/MS
Coccidiostats are veterinary medicine licensed to prevent and treat coccidiosis and histomoniasis, diseases that cause serious health and economic problems in several varieties of livestock. Normally, these compounds are administrated regularly via feed to the animals. Despite all the requirements during feed fabrication a certain percentage of coccidiostats remain in the production circuit and contaminate subsequent feed batches (Galarini et al., 2009). Due to the unavoidable carryover of these substances, the European Commission decided to set up maxim level of coccidiostats in non-target feeds (Regulation 574/2011). Since 2011 residues of lasalocid in non medicated feed should not exceed 1.25 mg/Kg in feed for dogs, calves, rabbits, equine species, dairy animals, laying birds, turkeys, chicken for fattening, for other species concentration of sodium lasalocid should not exceed 3.75 mg/Kg. The aim of this study was to develop a method for the reliable identification and quantification of residues of lasalocid (Fig. 1) feed samples.
Figure 1. Chemical structure of lasalocid.
Materials and methods
Chemicals, reagents and stock solutions
Lasalocid with purity greater than 98% was obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile and methanol were purchased from Scharlau Chemie (Barcelona, Spain). Formic acid (purity > 99% for analysis) was purchased from Acros Organics (Geel, Belgium). Demineralised water, resistivity higher than 18.0 MU, was obtained in-house with a Milli-Q water system (Millipore, Bedford, MA, USA). Standard was prepared by dissolving 250 mg of the substance in 250 mL of methanol. This solution was further mixed with acidified methanol to obtain solution at the following concentrations: 10, 20, 32, 40, 64, 100, 160 and 320 mg/L. A solution of robenidine-d8 used as internal standard (IS) was prepared by diluting 250 mg of in 250 mL of methanol. All of the standard solutions were stored in amber glass bottles at -18ºC for a maximum of three months.
HPLC-MS/MS conditions
The HPLC-MS/MS system consisted of an HPLC model 1100 from Agilent Technologies (Waldbronn, Germany) composed of a quaternary pump, a degasser, and an autosampler. The mass spectrometer (MS) was a QTrap 2000_ from Applied Biosystems/ MSD Sciex (Toronto, Canada) with an integrated TurboIonSpray for molecule ionization. The software, Analyst 1.4.1, also from Applied Biosystems/MSD Sciex (Toronto, Canada), was employed to acquire the data and control the system. Extracts (10 mL) were injected into a Synergi 2.5-mm Polar-RP
100 Å (50 x 2.0 mm) analytical columns used in conjunction with a Polar-RP (4.0 mm x 2.0 mm) SecurityGuard cartridge, both from Phenomenex (Macclesfield, UK). A binary mobile-phase composed of 0.1% formic acid in water (phase A) and 0.1% formic acid in acetonitrile (phase B), pumped at 150 mL min_1 for 36 min, was required for the complete elution of the analytes. The mobile-phase gradient profile was as follows: 0-2 min, 98% A; 2-3 min, 85% A; 3-4 min, 75% A; 4-8 min, 55% A; 8-9 min, 50% A; 914 min, 30% A; 14-25 min, 7% A; 25-28 min, 0% A; 28-32 min, 75% A; 32-36 min, 100% A. The flow rate was held constant at 0.150 mL/min. The MS parameters selected for the optimum detection of lasalocid were; precursor ion 613, first product ion 377, second product ion 595, declustering potential 126, entrance potential 11, collision-cell entrance potential 32, collision energy 69, cell exit potential 4.
Extraction conditions
Lasalocid was extracted from the feed samples employing 5 ml of acetonitrile followed by 10 min of sonication and 15 min of centrifugation at 1509 g on a centrifuge model 5415D from Eppendorf (Hamburg, Germany). The supernatant was collected and transferred into a clean graduated 10 mL tube and evaporated to dryness under a gentle stream of nitrogen at 45 ºC, re-dissolved in 0.1 mL of acidified methanol. Disposable 0.45 μm Durapore Polyvinylidene fluoride (PVDF) filters from Millipore (MA, USA) were used for extract filtration. Once filtrated, the extract was directly transferred to an amber glass HPLC vial (with a 0.3 mL insert vial), and stored at -18 ºC prior to sample analysis by HPLC-MS/MS.
Results and discussion
Figure 2 shows the MRM chromatogram of lasalocid in feed samples spiked with lasalocid at 100 μg/Kg.
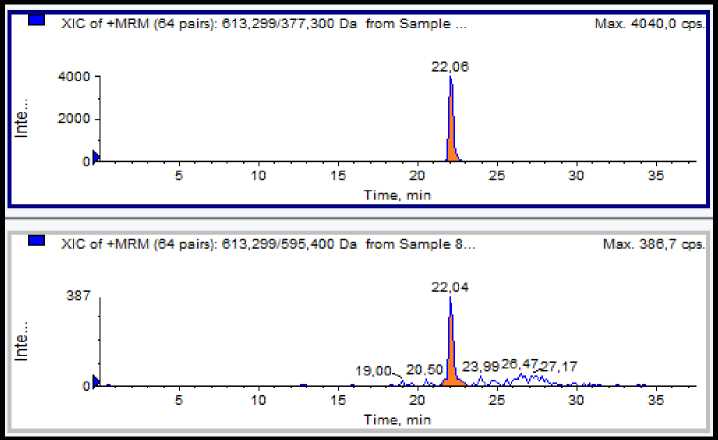
Figure 1. MRM chromatogram of a feed samples spiked with lasalocid at 100 μg/Kg.
Matrix-matched calibration curves were prepared for control and quantification purposes, 1g of sample was used for each calibration level. Feed samples were fortified by spiking them with different aliquots of a standard of lasalocid at 0, 50, 100, 250, 500, 1000 and 1500 μg/Kg (lasalocid). After fortification and prior to extraction, the samples were shaken and kept in the dark for one hour. To control the quality of the reagents and the procedure, two additional samples were prepared: a blank of reagents (all the reagents without feed) and fortified reagents (reagents spiked with lasalocid at the ML without feed). After the analysis of the quality control samples and the samples if the concentration of a sample was out of the calibration curve range it was diluted with acidified methanol.
Compared with other available methods the method presented here requires the amount of acetronitrile for the extraction of lasalocid from feed samples, this is an important aspect to consider as the price of this solvent is increasing each year due to its high demand. Fluorescent methods has being described such as the one described by Focht, 2008 and also HPLC-MS/MS methods which used different volumes of acetonitrile; 100 ml of a solution containing 85% of acetonitrile (Rokka et al., 2013), 40 ml (Vincent et al., 2011), 25 ml ( Cronly et al., 2011) and 12 ml (Galarine et al. , 2009). Overall, it could be said that the method presented uses the lowest amount of acetonitrile.
Conclusion
An easy and cheap HPLC-MS/MS method for the analysis of lasalocid in feed medicated and contaminated feed samples is presented. The detection is achieved for concentration between 60 and 1500 μg/Kg. The method full fills the criteria of the European Decision 2002/657/EC for the detection of lasalocid.
Список литературы Monitoring the presence of residues of lasalocid in feed samples HPLC-MS/MS
- Galarini, R., Fioroni, L., Angelucci, F., Tovo, G. R., & Cristofani, E. (2009). Simultaneous determination of eleven quinolones in animal feed by liquid chromatography with fluorescence and ultraviolet absorbance detection. Journal of Chromatography A, 1216. -Р. 8158-8164.
- COMMISSION REGULATION (EU) No 574/2011 of 16 June 2011 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto L 159. -Р. 7-24.
- Focht, C. (2008). Determination of Lasalocid Sodium in Animal Feeds and Premixes by Reversed-Phase Liquid Chromatography: Collaborative Study. Journal of AOAC International, 91. -Р. 479-488.
- Vincent, U., Ezerskis, Z., Chedin, M., Von Holst, C. (2011). Determination of ionophore coccidiostats in feeding stuffs by liquid chromatography-tandem mass spectrometry. Part II. Application to cross-contamination levels and non-targeted feed. Journal of Pharmaceutical and Biomedical Analysis 54. -Р. 526-534.
- Mark Cronly, M., Behana, P., Foleya, B., Maloneb, E., Shearanb, P., Regan, L. (2011). Determination of eleven coccidiostats in animal feed by liquid chromatography-tandem mass spectrometry at cross contamination levels Analytica Chimica Acta 700. -Р. 26-33.


