Optimization of the technological mode of processing of Karatau phosphorites on cyclic technology by method of mathematical planning of an experiment
Автор: Akhmetova S.O.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Естественные науки
Статья в выпуске: 4 (125), 2019 года.
Бесплатный доступ
In the article the results of studies of decomposition process of Karatau phosphorites by phosphoric acid are presented. Mathematical processing of the results of an experiment was carried out. Kinetic characteristics of the chemical reaction are determined, which can be used to evaluate technological parameters and select optimal conditions of phosphorite decomposition. The process of decomposition of phosphorites of different structure was optimized by the method of mathematical planning of the experiment. The obtained dependencies can be used for determination of decomposition parameters under production conditions in order to achieve the maximum process indicators.
Phosphates, chemical processing, monocalciumphosphate, phosphoric acid, insoluble rest, degree of decomposition, rate of decomposition
Короткий адрес: https://sciup.org/140249065
IDR: 140249065 | УДК: 661.15
Текст научной статьи Optimization of the technological mode of processing of Karatau phosphorites on cyclic technology by method of mathematical planning of an experiment
One of the urgent problems of development of the mineral fertilizers industry is providing it with the source of raw materials connected with involvement in processing of new types of phosphatic raw materials. Characteristic of natural phosphates of many fields is a low content in them Р 2 О 5 and rather high concentration of impurity which considerably complicate processing of phosphorites. Kazakhstan occupies one of the leading places in the world in size of explored reserves of natural phosphates [ 1 ] .
Phosphoritic ores of Karatau fields basin are subdivided into three main industrial types: rich, ordinary and poor. The rich phosphoritic ores are developed in superficial zones of a number of fields (Zhanatas, Kokdzhon, etc.), on average contain 28-30% Р 2 О 5 ; 7-10% SiO 2 , 4-8% CO 2 , 47% СаО, to 2,5-3,5% МgO, 1-1,5% Fe 2 O 3 , to 1% Аl 2 О 3 ; they are suitable for direct processing by acid methods. Ordinary phosphoritic ores contain 2125% P 2 O 5 . Poor phosphoritic ores are presented by siliceous and phosphatic breeds with the maintenance of 18-21% Р 2 О 5 , 25-30% SiO 2 , 5-7% CO 2 , 2-2,5% Fe 2 O 3 , 1-2% Al 2 O 3 , siliceous and slate ores contain 16-20% Р 2 О 5 and 30-40% SiO 2 [2].
Generalizing the said above, it is possible to allocate the next moments:
-
- Karatau phosphorites belong to refractory ores, generally carbonate or siliceous [2]. Their enrichment is directed to increase in maintenance of the main component - Р 2 О 5 and the maximum decrease in impurity, first of all carbonates of calcium and magnesium. A variety of the chemical composition and mineralogo-petrographic characteristics of Karatau phosphorites doesn't represent an opportunity to recommend for industrial realization any one rather universal and effective method of their
enrichment [3];
-
- At the process of ore enrichment a large amount of waste is formed [1];
-
- Processing of the low-quality phosphatic Karatau raw materials to extraction phosphoric acid is characterized by obtaining less concentrated acid (20-22% of Р 2 О 5 ) and a low exit of Р 2 О 5 in the final product (92-93%) [4].
Thus, now, the problem of rational, fuller use of raw material resources of Karatau is particularly acute very much: extraction of Р 2 О 5 from the ores to a useful product makes 76,2% [1], i.e. a quarter of the phosphorus-containing raw materials extracted from the deposit is excluded from the final product, is lost forever.
Works dealing with the development of technology to produce concentrated fertilizers using phosphates of new deposits are very urgent, the problem of using low-quality phosphorites being the most interesting. One of the most important aims of the problem being discovered is the search for ways for direct processing of low-quality and non-standard raw materials into high-quality products. The works dealing with studying speeds and mechanisms of decomposition of natural phosphates in pulps which do not thicken may be referred to as the most important in this respect. They helped to offer a set of new so to say cyclic ways to produce mineral fertilizers.
These circumstances have induced us to study a possibility of application of the recirculation phosphorus-acid method for processing into monocalciumphosphate and extraction phosphoric acid the Karatau basin off-balance phosphorites [6-7].
Research objects and methods
As an object of the researches 3 samples of phosphates of various fields of the Karatau basin have been taken. The chemical composition of the studied samples is given in table 1.
Table 1 – The chemical composition of the studied samples of Karatau phosphorites in mass. %
|
Samples of initial phosphates |
Maintenance of the main components |
|||||||
|
Р 2 О 5 |
CaO |
MgO |
Fe 2 O 3 |
Al 2 O 3 |
CO 2 |
F |
i.r. |
|
|
1. Carbonate ore of Zhanatas field |
21,03 |
36,03 |
2,4 |
2,99 |
1,02 |
8,78 |
1,93 |
19,86 |
|
2. Siliceous and slate ore of Koksu field |
15,37 |
23,52 |
0,99 |
2,79 |
1,91 |
1,08 |
2,08 |
49,15 |
|
3. Phosphatic and siliceous ore of Koksu field |
22,58 |
35,82 |
0,79 |
2,79 |
2,03 |
3,95 |
2,79 |
26,2 |
The previous researches of the authors on studying of the regularities of decomposition of low-quality phosphatic raw materials of Chilisay and Karatau fields in process with recirculation of phosphoric acid have shown that an optimum norm of the phosphoric acid with the concentration 36-42% of Р 2 О 5 fluctuates within 500-600% from stoichiometric necessary on formation of monocalciumphosphate [7-8]. This has given to the authors the base for carrying out experiments for the purpose of detection of the kinetic regularities of decomposition of Karatau phosphorites of various structure and quality phosphoric acid of concentration of 36% Р 2 О 5 (50% of H 3 PO 4 ) and norm of 550%.
Experimental kinetic data have been obtained in the periodic mode at the temperatures 75 ± 2 ° С and 95 ± 2 ° С by the technique described when studying kinetics of decomposition of Chilisay phosphorites by large excess of phosphoric acid [8].
For the description of the kinetics of decomposition Chepelevetsky and Drozdov's equations [9 are used:
At mathematical processing of the results of experiments methods of approximation of the results of experiments with use of polynoms 2-4 degrees, and also the equations of indicative, logarithmic and exponential regressions have been tested. Mathematical processing of the results is made by means of specially developed complex of the programs in the Mathcad-2001 system [10].
Studying kinetics of decomposition of all specified phosphorites phosphoric acid of one concentration (50% Н 3 РО 4 ) and at one norm (550%) isn't enough from the point of view of determination of the optimum parameters of the process taking into account specific features of raw materials therefore further research of influence of various factors on the process of phosphoric acid decomposition of low-quality Karatau phosphorites by method of planning of an experiment for the purpose of optimization of a cyclic technology of their processing was required.
The purpose of the given research was definition of optimum conditions of carrying out reaction of decomposition of the studied phosphorites phosphoric acid and creation of the generalized model considering the main indicators of the process. Researches are conducted with an application of the second order orthogonal plan method of planning of experiment with a star shoulder a = ± 1,72 allowing to define simultaneous influence of various factors on the process of decomposition of phosphatic raw materials.
Results and Discussions
Kinetics of the process of decomposition of the phosphorites phosphoric acid and mathematical processing of the experimental data
Results of the experiments are presented in table 2.
Table 2 – Results of the experiments on study of the rate of decomposition of the phosphorites
|
1 sample |
2 sample |
3 sample |
||||||
|
t, ° C |
T decom , min |
Decomposi tion degree a |
t, ° C |
T decom , min |
Decomposi tion degree a |
t, ° C |
T decom , min |
Decomposi tion degree a |
|
75 |
1 |
0,2115 |
75 |
1 |
0,3000 |
75 |
1 |
0,2275 |
|
75 |
2 |
0,3632 |
75 |
2 |
0,5500 |
75 |
2 |
0,4034 |
|
75 |
3 |
0,5328 |
75 |
3 |
0,8250 |
75 |
3 |
0,5517 |
|
75 |
4 |
0,6450 |
75 |
4 |
not defined |
75 |
4 |
not defined |
|
75 |
5 |
0,8600 |
75 |
5 |
0,9300 |
75 |
5 |
0,9300 |
|
75 |
10 |
0,8691 |
75 |
10 |
0,9660 |
75 |
10 |
0,9700 |
|
75 |
20 |
0,9125 |
75 |
20 |
0,9790 |
75 |
20 |
0,9800 |
|
75 |
30 |
0,9210 |
75 |
30 |
0,9899 |
75 |
30 |
0,9900 |
|
75 |
40 |
0,9420 |
75 |
40 |
0,9940 |
75 |
40 |
0,9915 |
|
75 |
50 |
0,9530 |
75 |
50 |
0,9955 |
75 |
50 |
0,9910 |
|
95 |
1 |
0,2270 |
95 |
1 |
0,3520 |
95 |
1 |
0,2400 |
|
95 |
2 |
0,3905 |
95 |
2 |
0,6000 |
95 |
2 |
0,4355 |
|
95 |
3 |
0,5642 |
95 |
3 |
0,8825 |
95 |
3 |
0,5800 |
|
95 |
4 |
0,6700 |
95 |
4 |
not defined |
95 |
4 |
not defined |
|
95 |
5 |
0,9000 |
95 |
5 |
0,9695 |
95 |
5 |
0,9500 |
|
95 |
10 |
0,9075 |
95 |
10 |
0,3755 |
95 |
10 |
0,9800 |
|
95 |
20 |
0,9200 |
95 |
20 |
0,9800 |
95 |
20 |
0,9860 |
|
95 |
30 |
0,9300 |
95 |
30 |
0,9840 |
95 |
30 |
0,9900 |
|
95 |
40 |
0,9480 |
95 |
40 |
0,9897 |
95 |
40 |
0,9937 |
|
95 |
50 |
0 9600 |
95 |
50 |
0 9955 |
95 |
50 |
0 9950 |
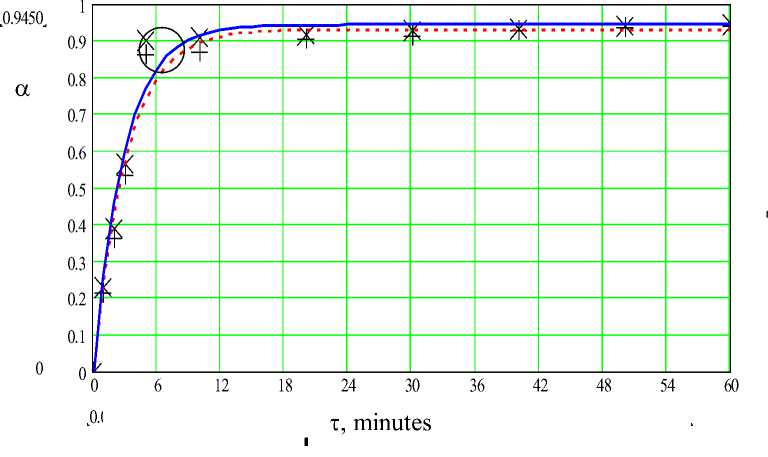
Continuous line and symbol X (experiment) - at a temperature 95 ° C, dashed line and symbol + (experiment) - at a temperature 75 ° C
Figure 1 - Dependence of degree of decomposition ( a ) on time ( t ) at N (H 3 PO 4 ) - 550%, C (H 3 PO 4 ) - 50% for the sample 1
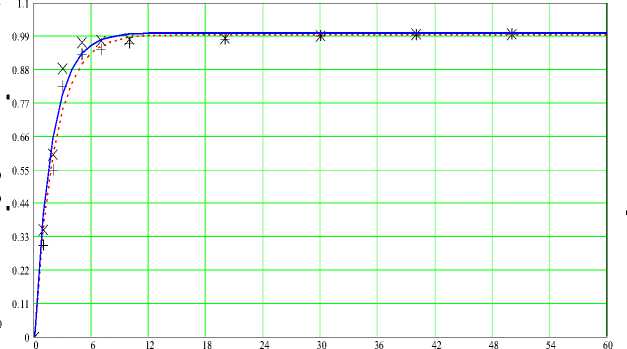
t , minutes
Continuous line and symbol X (experiment) - at a temperature 95 ° C, dashed line and symbol + (experiment) - at a temperature 75 ° C
Figure 2 - Dependence of degree of decomposition ( a ) on time ( t ) at N (H 3 PO 4 ) - 550%, C (H 3 PO 4 ) - 50% for the sample 2
Follows from the fig. 1 (Results of experiments for a sample 1) two stages of the process of decomposition of phosphorites are allocated (1 – actually a decomposition stage, 2 -a further decomposition stage). Distinction in a time of course of each stage of decomposition is rather big (3-7 min. – 1 stage; 50-55 min. – the 2nd stage). For the samples 2 and 3 (fig. 2-3), in general, the nature of dependence of degree of decomposition on time is similar.
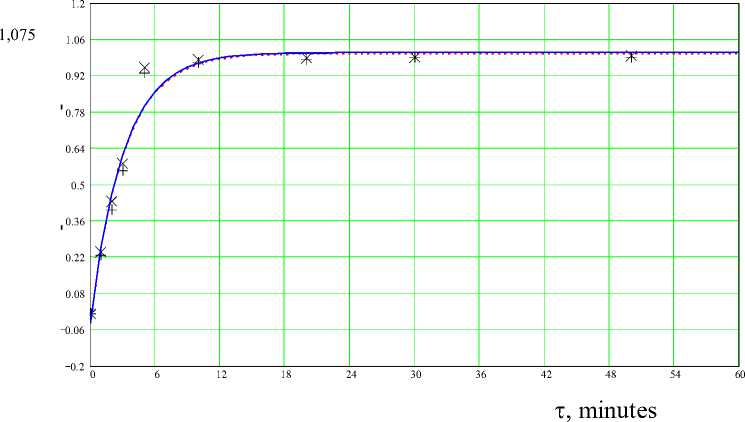
Continuous line and symbol X (experiment) - at a temperature 95 ° C, dashed line and symbol + (experiment) - at a temperature 75 ° C
Figure 3 - Dependence of degree of decomposition ( a ) on time ( t ) at N (H 3 PO 4 ) - 550%, C (H 3 PO 4 ) - 50% for the sample 3
Application of methods of а ( т ) approximation mathematical expression has had an opportunity of obtaining reliable a ( t ) values in any intermediate points of time of the studied range. Besides, it has given the chance to obtain an analytical expression of rate of reaction V( t ) .
The best indicators of adequacy of mathematical modeling and reflection of a quantitative nature of kinetic regularities are obtained when using exponential regression of a look:
a a = a • exp( b • t ) + c (1)
Continuous and dashed curves in fig. 1 are results of approximation of experimental data for a sample 1 for all range of time expressions at a temperature 75 ° C:
a a = - 0.9633 • exp( - 0.323 • t ) + 0.9202 (2)
and at a temperature 95 ° C:
a a = - 0.9783 • exp( - 0.3475 • t ) + 0.9895 (3)
Coefficients of Pearson correlation for expressions (2) and (3) are respectively equal 0.9902 and 0.9845 that speaks about high precision of approximation.
Predicted rate of reaction when using dependence (1) is defined as its derivative:
d a a d T
= a • b • exp(b • t ) + c
In spite of the fact that the correlation coefficient at approximation is high, a value at time t = 5 minutes (it is led round by a circle) as if drops out of dependence. The similar picture is observed also in experiments with other samples. It means that, since timepoint of 4 - 7 min., there is a quick transition from the first stage to the second. Therefore the most real results of reflection of a qualitative picture of kinetics of the process can be obtained by separate consideration of the first stage, i.e. actually decomposition stages.
In work [11] it is also shown that at dissolution of phosphates in phosphoric acid a uniform hypothesis (uniform on a surface and by the characteristic size) of the nature of a process of dissolution of a conditionally spherical particle at degree of dissolution a higher than 92% isn't confirmed. Considering these circumstances for determination of kinetic parameters the experimental data corresponding to the first stage of decomposition are used.
The carried-out mathematical processing of the results of the experiment has allowed to calculate a reaction order, a constant of the rate of reaction and an apparent energy of activation of the process of The values of constants a, b, c and kinetic decomposition for all three samples of phosphorites. parameters of the process are given in table 3.
Table 3 – Results of mathematical processing of experimental data
|
0 ^ ■pH СЛ |
и 0 (D 5 Ph 8 |
Coefficients of equation (1) |
Correlation coefficient R |
Reaction order n |
Constant of reaction rate K |
Apparent energy E, kJ/mol |
||
|
a |
b |
c |
||||||
|
1 |
75 |
-1,3630 |
-0,1607 |
1,3646 |
0,9993 |
0,5887 |
0,2060 |
7.1948 |
|
95 |
-1.2337 |
-0.1974 |
1.2344 |
0.9994 |
0.6757 |
0.2283 |
||
|
2 |
75 |
-5.7332 |
-0.0513 |
5.7362 |
0.9996 |
0.1054 |
0.2882 |
9,5162 |
|
95 |
-2.8349 |
-0.1221 |
2.8406 |
0.9990 |
0.2131 |
0.3301 |
||
|
3 |
75 |
-1.1405 |
-0.2195 |
1.1411 |
0.9999 |
0.8017 |
0.2436 |
6,2200 |
|
95 |
-1.1137 |
-0.2464 |
1.1130 |
0.9999 |
0.8270 |
0.2663 |
||
The found values of activation energy correspond to the process going mainly in the internal diffusive area that confirms the mechanism of decomposition of phosphorites with phosphoric acid stated above.
Intensification of the process of decomposition at a circulating way of obtaining a monocalciumphosphate from Karatau phosphorites.
When developing the phosphoric-acid cyclic method of processing the Karatau phosphorites of various structure and quality it is revealed that in some cases carrying out the process is complicated considerably by the low speed of a filtration of the insoluble rest [5-6; 8].
In actual practice decomposition of phosphorites is made phosphoric acid which, depending on a way of its producing and concentration, contains a quantity of sulfuric acid. Interaction of natural phosphate with free sulfuric acid leads, on the one hand, to increase in reactionary activity of a liquid phase of a pulp, with another – crystallization and sedimentation of calcium sulfate on the surfaces of grains of natural phosphate can exert the braking impact on decomposition process [8].
As target output variables (exits – vector Y ) for each of the three studied samples of phosphorites are chosen:
Y i - rate of decomposition [ - ] ;
-
Y 2 - speed of filtration of the insoluble rest (i.r.) on a dry deposit [ kg/m2 - h ] ;
-
Y 3 - yield of the monocalciumphosphate [ g from 50 g of phosphate material ] .
Input parameters (factors - vector X ) were:
X i - temperature of decomposition [° C ] ;
X 2 - time of decomposition [ min ] ;
X 3 - concentration of phosphoric acid [ % P 2 O 5 ] ;
X 4 - norm of phosphoric acid [ % ] N;
Х 5 - content of sulfuric acid in phosphoric acid [ % ] .
Significant coefficients b of the mathematical model which is adequately describing process of decomposition of phosphorites, having a regression equation appearance have been defined:
Y = b0 + b1 . X1 + b2 • X2 + b3 • X3 + b4 • X4 + b5 • X5+
+ b12 • X 1 • X2 + b13 • X 1 • X3 + b14 • X 1 • X4 + b15 • X 1 • X5 +
+ b23 • X2 • X3 + b24 • X2. X4 + b25 • X2. X5 + b34 • X3 • X4 +
+ b35 • X3 ' X5 + b45 ' X4 ’ X5 + + b11 ’ X2 + b22 ' X2 + b33 " X2
+ b 44 • X 42 + b 55 • X 2
Where Y - calculated value of exit.
In general view coefficients ( i • j ) of the equations were required:
Y^ y = f(5 c ,b ) , (6)
Where i – number of a sample of phosphorite;
j – number of an exit of Y (for the Y 22 researches weren't conducted);
— required B - vector of the coefficients of regression equation.
The ranges of change of the factors chosen on the basis of the analysis of the studied system are presented in table 4.
Table 4 – Areas of the independent variables research
|
Factors |
X 1 |
X 2 |
X 3 |
X 4 |
X 5 |
|
LOW LEVEL ( - 1) |
80 |
15 |
38 |
450 |
1,05 |
|
High level (+1) |
95 |
45 |
42 |
550 |
3,95 |
|
Zero level (0) |
87,5 |
30 |
40 |
500 |
2,5 |
|
Variability interval ( A X) |
7,5 |
15 |
2 |
50 |
1,45 |
|
Arm + a |
100,39 |
55,78 |
43,44 |
585,94 |
4,99 |
|
Arm - a |
74,61 |
4,22 |
36,56 |
414,06 |
0,01 |
The program for the personal computer on the basis of the Ms Excel system – 2000, used in the Windows-2000 system environment is developed for automation of processing of the results. It has allowed to obtain almost instantly during dialogue all necessary results of calculations and to provide search of the optimum modes of carrying out reaction of phosphorites decomposition.
Exception of the described results is experience for Y23 (the second sample - siliceous and slate ore of Koksu field, 15,37% Р2О5) since the obtained mathematical model in this case was inadequate. After the additional analysis it is found out, for this case factors Xi (t0,C) and X2 (t, minutes) have no significant effect on Y23 (yield of the monocalciumphosphate). Therefore for this sample the complete factorial experiment (CFE) 23 (8 experiences) for the purpose of definition of dependence of У23 on (Х2; Х4; Х5) has been made.
The CFE mathematical model is used in the regression equation form:
Y = bo + b, ■ X3 + b4 ■ X4 + b5 ■ X5 + b34 ■ X3 ■ X4 + »35 ■ X, . X5 +
+ b45 ' X4 ' X5 + b345 " X3 " X4 * X5
at ranges of change of the factors presented in table 6.
Table 6 –Areas of the independent variables research at CFE for definition of dependence of Y 23 on Х 2 ; Х 4 ; Х 5
|
Factors |
X 3 |
X 4 |
X 5 |
|
LOW LEVEL ( - 1) |
38 |
450 |
1,05 |
|
High level (+1) |
42 |
550 |
3,95 |
|
Zero level (0) |
40 |
500 |
2,5 |
|
Variability interval ( A X) |
2 |
50 |
1,45 |
The
L x max .-. ——
expected values Y min J for the optimum and ranges modes are a
priori known. The developed programs of processing and research of the results of the experiment allow count very quickly parameters of mathematical model (5) and an exit Y .
The analysis of the results of mathematical modeling has shown that change of a temperature of decomposition and norm of Н3РО4 in the studied ranges practically don't influence degree of decomposition, process of decomposition comes to an end in 30-40 min., depending on quality of raw materials. Degree of decomposition of phosphorites decreases a little with increase in concentration of phosphoric acid and amount of sulfuric acid in mix.
However even the insignificant content of sulfuric acid (from 0,2 to 0,5%) increases the speed of a filtration of the i.r. by 3-3,5 times (from 55 to 160 kg/m2-h on a dry deposit at decomposition of the 1st sample, from 89 to 250 kg/m2-h on a dry deposit at decomposition of the 3rd sample), without having significant effect on degree of decomposition of phosphorites. Further increase in content of sulfuric acid in phosphoric acid leads to decrease in degree of decomposition by 3-6%, depending on concentration of phosphoric acid, and also to sharp reduction of the yield of the monocalciumphosphate. Use for decomposition of less concentrated phosphoric Thus, the following optimum modes have acid leads to some reduction of the yield of the been defined:
monocalciumphosphate as well.
|
factors samples |
X 1 t, ° C |
X 2 т , min |
X 3 С Н 3 РО 4 ,% |
X 4 N H 3 PO 4 ,% |
X 5 C H 2 SO 4 ,% |
|
1 |
95 |
40 |
41 |
550 |
0,25 |
|
2 |
90 |
30 |
40 |
550 |
0,3 |
|
3 |
95 |
40 |
39 |
500 |
0,7 |
At the same time it is possible to carry out process of decomposition with rather high degree (0,95-1,00) when the yield of the monocalciumphosphate is equivalent on the maintenance of Р 2 О 5 to amount of the entered phosphatic raw materials. However even though the speed of a filtration of the i.r. increases a little, but is insufficient for the technological purposes.
Conclusions
The process of decomposition of the poor phosphate raw materials of three fields of Karatau basin with a large excess of phosphoric acid concentration of 40% Р 2 О 5 was studied.
New data on kinetics of decomposition of poor Karatau phosphatic raw materials of three fields 5,5-multiple excess of 40% Р 2 О 5 concentration phosphoric acid are obtained. It is established that the main opening of ores happens the first 5 minutes of decomposition to the maximum speed, in the next 30-40 minutes there is futher decomposition of the raw materials to achievement of almost 100% degree of decomposition. Lower degree of decomposition of carbonate phosphorite (0,96) is connected with the increased maintenance of MgO in this ore.
It is established that in the range of temperatures 348-368K temperature have no significant effect on a degree of decomposition, especially at the 2nd stage of the process. Almost full decomposition of ores is provided with high activity of hydrogen ions in a liquid phase of suspension, and also lack of again formed firm phase.
Mathematical processing of the results of the made experiment is carried out, the regression equations which are adequately describing dependences of speed and degree of decomposition on process parameters, and also kinetic characteristics of a chemical reaction behavior which can be used for assessment of technological indicators and the choice of optimum conditions of decomposition of poor Karatau phosphorites are obtained. Optimization of the process of decomposition of phosphorites of various structure is performed by method of mathematical planning of an experiment.
The obtained dependences can be used for the determination of the parameters of decomposition under production conditions for the purpose of achievement of the maximum indicators of the process.
Список литературы Optimization of the technological mode of processing of Karatau phosphorites on cyclic technology by method of mathematical planning of an experiment
- Timchenko A.I., Krasnov A.A., Romanov V.L. Resources of phosphatic raw materials of the Karatau basin for chemical processing. // In book: Works of NIUIF, M.: Issue 255. - 1988. Increase in efficiency of the phosphorus-containing fertilizers produced on the basis of Karatau phosphorites.[In Russian]
- Bliskovsky V.Z. Material structure and enrichability of phosphorite ores. M.: Nedra, 1983. PP. 56-60. [In Russian]
- Shokhin V.N., et al. Floatation and chemical enrichment of phosphatic ores. M.: Nedra, 1991. - 205, [1] p. [In Russian]
- Zabeleshinsky Yu.A.,et al. Quality of phosphatic Karatau raw materials and its influence on technology of extraction phosphoric acid // Survew inform. Ser. "Mineral fertilizers and sulfuric acid". M.: NIITEHIM,1986. - 30 p. [In Russian].
- Akhmetova, S.O., et al. Patent of RK No. 4997. Way of obtaining mineral fertilizer. Bulletin "Industrial Property", 2001, No. 3. - 3p. [In Russian].
- Akhmetova S.O., et al.Patent of RK No. 10423. Way of obtaining extraction phosphoric acid. Bulletin "Industrial Property", 2006, No. 4. -3p. [In Russian]
- Akhmetova S.O., Shapiro L.D., Moldabekov Sh.M. Kinetics of decomposition of Chilisay phosphorites phosphoric acid // Complex use of miner. raw materials, 1992. - No. 11.- PP. 29-32. [In Russian]
- Moldabekov Sh.M., Akhmetova S.O. Technology of processing of substandard Karatau phosphorites on monocalciumphosphate // Scientific and Technical Journal "News of Science of Kazakhstan". - 1999, - No. 2. - PP. 11-15. [In Russian]
- Akhnazarova S.L., Kafarov V.V. Methods of optimization of an experiment in chemical technology, M.: The Higher School, 1985. - 327 p.[In Russian]
- Dyakonov V.P., Abramenkov I.V. Mathcad 7.0 in mathematics, physics and in the Internet. M.: Knowledge,1998. - 345 p.[In Russian]
- Petropavlovsky I.A., et al. // Works of Moscow Chemical-Technological Institute after D.I. Mendeleev.Issue. 132. 1984.PP. 38-43. [In Russian]


