Silvering and coppering of chemically inert textile materials by means of wet-chemical process
Автор: Onggar T.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Технология текстиля и одежды, дизайн
Статья в выпуске: 1 (135), 2022 года.
Бесплатный доступ
The development of the wet-chemical silvering and coppering method on inert fiber surfaces and a number of usable materials (silver, copper and others) allows fabrication of several textile structures with functional characteristics. In order to analyse the silver and copper coating characteristics such as structure, homogeneity and crack formation, the surface morphology (SM) was investigated using the scanning electron microscopy (SEM). The chemical structuring of the surfaces (amount of carbon, oxygen, nitrogen, silicium, copper and silver) is analysed with an energy-dispersive x-ray spectroscopy (EDX). Phase formation and crystalline properties of the films were investigated by X-ray diffraction (XRD) with a Cu-Kα radiation reflection geometry source. Furthermore, textile-chemical analyses for the wash fastness were carried out as well.
Silvering, coppering, wet-chemical process, metallization, textile material, sensor materials, antibacterial
Короткий адрес: https://sciup.org/140293859
IDR: 140293859
Текст научной статьи Silvering and coppering of chemically inert textile materials by means of wet-chemical process
The metallization of non-conductive textile material has gained more importance over the last few years. Metallized textile products have electrical- and heat-conductive, electro-magnetically shielding, sensory, microbicidal, fungicidal and anti-allergenic characteristics. The antimicrobial and fungicidal effect increases in the rows Fe
Heinlaan et al., tested the toxicity of nanoparticles (NP) of ZnO, CuO and TiO2 for Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus with a special emphasis on metal oxide formulations (nano or bulk) and hydrolysis of metal oxides. That metal oxide particles do not necessarily have to enter the cells to cause the toxicity [7]. That stable large specific surface area CuO photo catalysts were effective in the inactivation of E. coli [8]. CuO nanoparticles are uniformly deposited onto the surface of a cotton bandage by the sonochemical method [3]. The silver ion and copper ion release property of silvered materials depends on different factors, such as the size of the metal particle [9], the thickness of the silver or cooper layer, the surface (smooth or porous) [9], and the drying temperature. The silver ion and copper ion release property of silvered materials also depends on additional external influential factors such as the fluid temperature, the water hardness and the concentration of chlorine in water. The silver layer or silver nano particles treated with very thin (~ nm) TiO2 layer [1, 9, 10], with a silica glass (Al/Ag≥1) [11], with a thin SiO2 film [12] or Ni-removed Ag-CNTs layer (carbon nanotubes) [13] were used in order to create a stable, controlled release of silver ions, therefore an efficient and durable antibacterial efficiency of metalized materials or nano composite materials suitable for future bacterial and biomedical applications. Other researchers utilized porous soft SU-8 matrix or meso porous layer to immobilize stable silver nanoparticles permanently [14].
The polyethylene terephthalate (PET) textile materials are metalized according to the current state of the art technology by separating the metal coatings from the vapour phase (Physical Vapour Deposition (PVD) [15, 16] and Chemical Vapour Deposition (CVD)), galvanically electrolytic (Electrolytic Deposition of Inorganic Coatings) or galvanically electroless (Chemical Deposition of Inorganic Coatings) coating [17-19], via plasma-technology [20] and wetchemical method [21-25]. PET textile materials or PET with cotton blend fabric were silvered as yarn, knitted fabric and woven fabric. The application fields of such functional textile material are from the medical field [3, 8, 26], the food equipment [3], the domestic cleaning [3], the safety field, sport and leisure field [27] to lightweight engineering and automotive engineering [28]. Explicit applications are among others medical textiles with inserted sensors or electrode systems for the recording, control and stimulation of vital functions, clothing textiles with integrated microchips for the identification of the material, protective textiles with inserted conductive sheets to increase the electrostatic charge, safety textiles with sensors or conductive sheets for the load and wear-out control in wire rope systems, heating textiles with integrated electrically heatable textile heating systems for the creation of heat, automobile textiles with inserted sensor systems in bedding to record the seat occupancy and position, spacer textiles with anti-microbial, air-permeable, pressure-elastic and drapable characteristics for the reduction of the settlement and reproduction of microorganisms in water and other fluidcontaining systems, smart textiles with highly conductive textile thread material for translucent light protection systems for museums, fiber-reinforced composites with integrated textilebased sensor networks for the damage-free structural control and for the measurement of different chemical/physical parameters of the components, textile flooring with light elements connected to each other (e.g. LED) for the signalization of emergency exits and luminous textiles with inserted LED-threads for the lightening of the car interior [29-32].
The demand of metalized synthetic fiber such as PET has been steadily increased for the application in water technology, electronics and sensors. PET fiber shows better hydrolysis stability than natural fibers or nylon (polyamide 6.6).
These textile materials need to be fulfilled the requirements for end use properties in different applications. The final properties e.g. electromagnetic shielding (≤109 ohms), electrical conductivity (≤105 ohms), thermal conductivity (for Ag 429 W/(m*K) and for Cu 401 W/(m*K)) and antimicrobial activity (for Ag 100 µg/l and for Cu 2000 µg/l in water) could be created on polyester.
The aim of this work is the development of a wet-chemical method for the reproducible metallization of different textile material to replace cost-intensive processes such as PVD, CVD or plasma technology. Especially, the intersections of the yarns demonstrate as weak points in the textiles during the deposition of closed PVD layer [15]. In the CVD method, high temperature treatments are necessary to obtain stable metal layers on the substrate. It provides difficulties for the metallization of PET fiber materials. The electrolytic deposition and electroless plating methods require several steps (pre-cleaning, rinsing, etching, rinsing, sensi-bilisation, rinsing, activation, rinsing, electroless plating, rinsing, drying and post-treatment), as well as tightly controlled process parameters (concentration of metal and reducing agent, pH, temperature, time etc.) [33-35]. Electroless metal deposition needs a high consumption of chemicals (sulfuric acid or chromium, palladium, formaldehyde, sodium dithionite etc.), which are ecologically hazardous. The tank must be coated with a sizing agent to the points at which they come into contact with the processing solution. The process must be shut down for several days to remove these deposits [15]. The electroless metal coated PET-fabric is after treated with polyurethane to enhance the electrical life of the metal layers [36]. The metal solution need to be constantly moved in order to deposit a homogenous copper layer on the paraaramid fiber surface so that no bubbles could be formed on the fiber surface [37]. The abovementioned disadvantages of the metal coatings were reduced or even avoided with the newly developed one-bath method. The challenge here is to deposite possibly homogenous and defect-free metal coatings with a good adhesion on multi- or monofilament fiber surfaces. The developed method should be flexible and applicable for all textile material in different forms (yarn, textile fabric and spacer fabric) to open up a wide application field for the metallized textile material in the future. By developing the wet-chemical silvering process, the use of oxidizing agent such as per sulfate in the presence of alkali metal hydroxide which leads to damage the textile material, could be waived. The addition of a brightener, binders, etching, stabilizing or sensibilizing agent which are essential for bonding and stabilization of the metal layer, can also be omitted. Besides, the silvering process is carried out at a low temperature (35 °C±3 °C). Therefore, the developed method is economical, ecological and environmentally friendly and easy technology than the previously known methods.
Materials and Research Methods Materials. In close cooperation with the textile company Sankt Micheln Co., Ltd. (Mülsen, Germany), different structures such as continuous filament yarns, textile fabrics and spacer fabrics made of PET were used as a substrate for metallization.
-
• The continuous filament yarn (T tex =48.3 tex, 34 single filament, without optical brightener, with round filament cross-section) was used as yarn.
-
• A fine warp knit fabric right/left, locknit, washed and fixed, T=50 dtex, 24 single filament, 12 stitch courses per cm was used as textile fabric.
-
• A spacer fabric: distance between two top surfaces is 16 mm.
Sodium hydroxide (Merck KGaA, Darmstadt, Germany) and Invadin LUN (CIBA, Basel, Switzerland) were used for the surface activation of the textile materials. In order to increase the wettability of the fiber, Invadin LUN, 4-tert-octylphenyl (polyethylene glycol-[9/10]) ether surfactant which acts as a universal wetting and deaerating agent was chosen for textile treatment liquors. For the specific coating, amino silane such as N-(2-Aminoethyl)-3-aminopropyltrimethoxysilane (Fluka Chemie GmbH, Oberhaching, Germany), aliphatic amine tetraethylenepentamine (Fluka Chemie GmbH, Oberhaching, Germany) and natural amine chitosan (Sigma-Aldrich Chemie GmbH, München, Germany) were chosen. The silver diamine complex was formed from silver nitrate (Grüssing GmbH, Filsum, Germany) and ammonia (VWR Prolabo GmbH, Darmstadt, Germany) solution (25 %). L(+)-ascorbic acid (Ap-pli Chemie GmbH, Darmstadt, Germany) was used as the reduction agent. Copper(II)-chloride-di-hydrate (Grüssing GmbH, Filsum, Germany), ethylene diamine tetra acetic acid (J. T. Baker Chemie, Munich) and N,N,N`,N`-tetrakis(2-hydroxypropyl)-ethylene diamine (Sigma-Aldrich Chemie GmbH, Steinheim) were utilized for the coppering.
Analysis Methods. Surface Characterization by means of Scanning Electron Microscopy. The morphological analysis of PET-fiber surfaces was carried out using a scanning electron microscopy (SEM) DSM 982 Gemini from the company Carl Zeiss AG (Jena, Germany). The test samples were examined with a magnification of 5000, 10000, 20000 and 50000.
Elementary Analysis. By the combination of SEM with an energy-dispersive x-ray spectroscopy (EDX), the percentage of the elements such as carbon (C), oxygen (O), nitrogen (N), silicium (Si), silver (Ag) and copper (Cu) was semi-quantitatively determined with the point analysis and the data was recorded.
Characterization of the thin films. Phase formation and crystalline properties of the films were investigated by X-ray diffraction (XRD) using a PANalytical X’Pert PRO (Almelo, Netherland) analyzer with a Cu-Kα radiation reflection geometry source. The scanning range of 2θ was from zero to 80° with a step size of 0,005 for silver and of 0,015 for copper.
Silver Analysis. In order to determine the mass-related percentage of silver (%) and copper on surface of the textiles, the test samples were ashed in the muffle furnace Controller B170 from the company Nabertherm GmbH (Lilienthal, Germany) at 900 °C±2 °C for 2 h and then the silver and copper concentration was quantified using atomic absorption spectroscopy (AAS) from ZEEnit 700 Company An-alytik Jena AG (Jena, Germany).
Determination of the Adhesion of the Silver Layer. In order to determine the adhesion of the silver coating, the DIN EN 20 105-C03 regulation was taken into consideration. The test sample was treated with a laboratory beaker dyeing apparatus from the company Werner Mathis AG (Zurich, Switzerland) for 30 min at 60 °C±2 °C. The weight loss was determined and the color of the liquor was visually evaluated.
Method Development for the Metallization of Chemically Inert Fiber Material. The metallization process was conducted in accordance with Figure 1 to fulfil the objectives of this study.
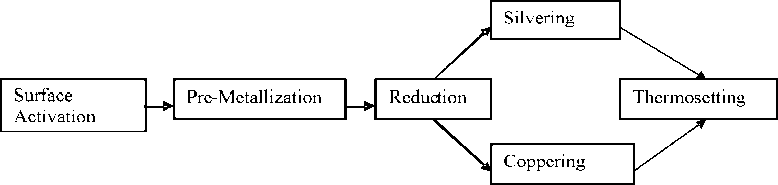
Figure 1 -Schematic illustration of the metallization process
Surface Activation. To activate the fiber surface of the PET-textile material, which needs to be metallized, it was pre-treated with a 0.5 N sodium hydroxide solution and one drop of the wetting agent Invadin LUN with a liquor ratio of 1:20 at 100°C for 30 min in a Labomat BFA-12 (Werner Mathis AG, Zurich, Switzerland) and afterwards rinsed with warm water until a pH-value of 6.0-7.0 was reached.
Pre-Metallization. In order to attach the silver diamine complexes on the chemically inert PET fiber surface, the pre-treated fiber surface was also pre-metallized with the newly developed one-bath method in a silver diamine complex solution at 35°C±2°C for 1 h. It is typical for the one-bath method that the finish-ing/coating agent (amino silane, natural or synthetic amine) is putted in one bath with a silver salt (AgNO 3 or Ag(NH 3 ) 2 NO 3 ) solution.
Reduction. After fixing the ionic silver on the fiber surface, the reduction was carried out with a 10% ascorbic acid solution at room temperature for 15 min to convert into metallic silver.
Wet-Chemical Silvering. The premetallized and reduced PET-textile material (continuous filament yarn, warp knitted fabric and spacer fabric) was then silvered at room temperature for 10 min in a aqueous Ag(NH 3 ) 2 NO 3 -solution, which was prepared with AgNO 3 - and NH 3 -solution. The silvered materials were once again reduced without intermediate drying with a 10 % ascorbic acid solution, then rinsed with cold water and dried at room temperature.
Wet-Chemical Coppering. The wet-chemically pre-silvered PET-textile material (continuous filament yarn, warp knitted fabric and spacer fabric) was coppered with stirring at 50°C±3°C in a aqueous copper-containing solution (3 g/l copper(II)-chloride-di-hydrate, 0.107 mol/l glyoxyl acid solution, 0.1 mol/l N,N,N`,N`-tetrakis(2-hydroxypropyl)-ethylene diamine (EDTP) or 0.067 mol/l ethylene diamine tetra acetic acid (EDTA) solution) until the test sample was fully covered with an adhesive light red copper coating. The coppered samples were then rinsed and afterwards dried at room temperature.
Thermosetting. After the silver-ing/coppering, the metallized PET textile material was fixed in a thermoset way in a Mathis-Labdryer TYP „LTE“ (Werner Mathis AG, Zurich, Switzerland) at 200°C for 2 min with tension of 19.62 N.
Application of the Developed WetChemical Metallization Methods.
Yarn Metallization. The texturized PET continuous filament yarn has been crosswise spooled in a “wild” winding with a 20° winding angle onto a cylindrical dyeing bobbin. A yarn dyeing apparatus (Zeltex Colorstar CS2 dyeing apparatus, Zeltex AG, Basel, Switzerland) was used for the wet-chemical pre-silvering, silvering or coppering. In this apparatus, the metallization of the bobbins was realized with a loading weight of 70 g using a one-bath method (exhaustion process). Here, the liquor was pumped through the bobbin at 35°C±2°C for 1 h with the changing of liquor flow direction (outsider-inside 9 min and insideroutside 11 min).
Metallization of Textile Fabrics. The pretreated warp knit fabric was dipped in a silvering solution and padded in a Lab-Padder TYP „HVF“ (Werner Mathis AG, Zurich, Switzerland) at 3*105 Pa pressure with 3 m/min speed. Because of the squeezing, a good adhesion of the remaining silver and an even distribution of the silver particles are guaranteed. After the pre-metallization of the warp knit fabric, the silvering or the coppering has been performed using bath exhaustion method.
Metallization of Spacer Fabrics. The pretreated spacer fabric was pre-metalized by dipping and then silvered or coppered using the bath exhaustion method.
Results and their Discussion
Surface Characteristics and attachment Possibilities of the Applied Coating on the Fiber Surface and Complex Formation of Functional Groups with Silver or Copper.
Yarn Metallization. Complete silver and copper coatings with a uniform silver particle distribution could be achieved on the fiber surface by systematic analyses of all process parameters and their optimal adjustment. After the pre-silvering of the PET continuous filament yarn using the wetchemical one-bath method, a dark grey color appeared on the fiber surface (Figure 2b). After the second silvering, the fiber surface turned golden (Figure 2c). The size and the shape of the silver particles, the distance between the silver particles, their storage and the way of filling the space are the reasons for the different colors. During the wet-chemical coppering, a light red color appeared on the PET-fiber surface (Figure 2d). The microscopic pictures show that the entire fiber surface was completely covered with a silver and copper coating.
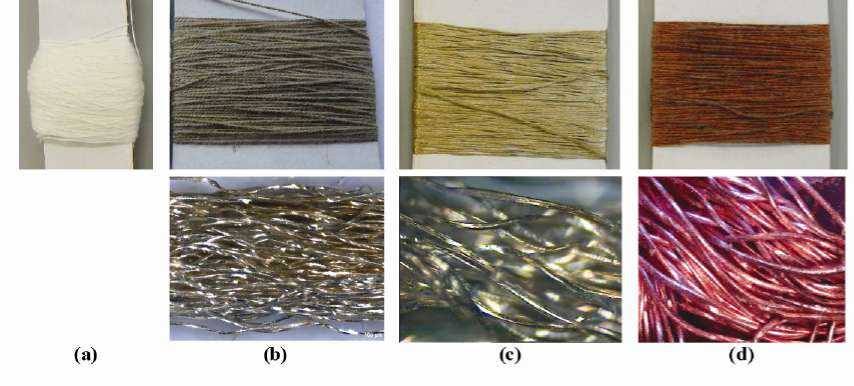
Figure 2 (a) Untreated, (b) pre-silvered, (c) silvered and (d) coppered PET continuous filament yarn
The surface morphology (SM) of the original and alkaline peeled continuous filament yarn was analyzed using the scanning electron microscopy (SEM) (Figure 3). PET fiber is inert on the view point of textiles which means that the fiber shows hydrophobic and non-polar character.
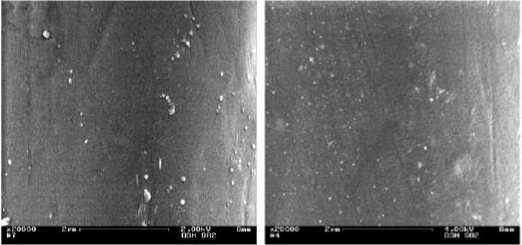
Figure 3 - SEM-images (x20.000) at (left) original and (right) with sodium hydroxide pre-treated of PET-continuous filament yarn
The sodium hydroxide (NaOH) solution slowly enters into the outer PET-fiber surface. This way, the NaOH-solution destroys the amorphous areas of polyester and can create fine micro holes or caverns (voids) on the fiber surface (Figure 3, right). At these places, the silver (Ag) particle is deposited which could be found on the fiber surface (Figure 4). Possibility for the adhesion of the silver on the substrate is the mechanical anchoring. Based on that process, the silver coating has been developed on PET surface.
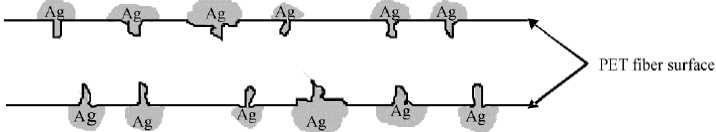
Figure 4 - Exemplary layout of the mechanical anchoring of Ag particle on the PET fiber surface after the premetallization
The alkaline treatment is commonly used for polyester in textile industry. A weight loss of the polyester by alkaline treatment of about 6-10 % is optimal for the fiber surfaces sufficiently roughen, not reduce the ultimate tensile strength so far. The tensile strength before and after the alkaline treatment and after the silvering are subject to future works.
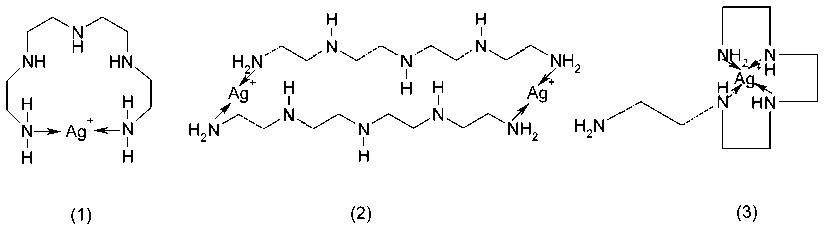
Scheme 1 Complex formation possibilities of silver ions with aliphatic tetraethylenpentamine (C 8 H 23 N 5 ).
The SEM-image (Figure 5a) clearly shows that the test samples pre-silvered with an [Ag(C8H23N5)n)]+-complex do not have a completely covered Ag-layer and therefore also have a lower silver percentage (5.45 % wt.) (Table 1). Such metal coatings show no electrical or heat conductivity. One reason was that the formation of the metal coating started in the reductive bath at those places of the polymer on which the noble metal nucleus was situated. Hence, small islands form, which grow into a closed metal layer step by step. This incompleteness of the metal coating was rectified through the second wet-chemical metallization process and an even spherical silver particle distribution and a completely covered silver layer on the fiber surfaces was determined using the scanning electron microscopy (Figure 5b). The results of the SM (Figure 5c) of coppered PET continuous filament yarn (with a [CuC10H12N2O8]2--complex solution) showed that crystalline triangular or polygonal copper particles were formed on the fiber surface and the surface was fully covered.
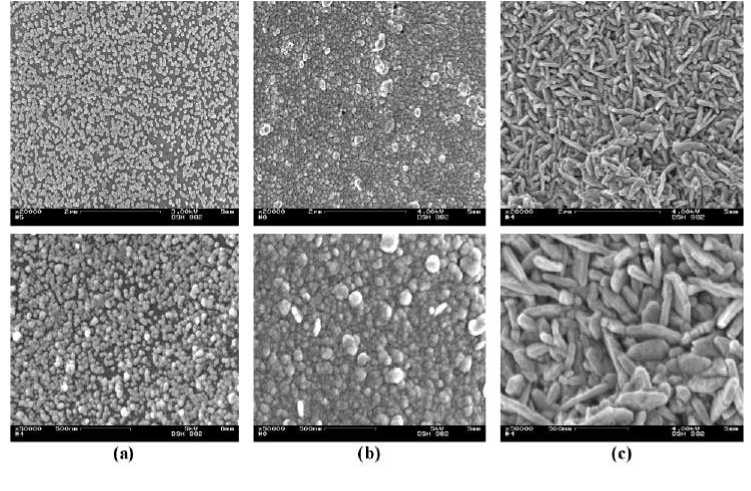
Figure 5 - SEM-images (x20.000 and x50.000) of (a) pre-silvered, (b) silvered and (c) coppered PET continuous filament yarn
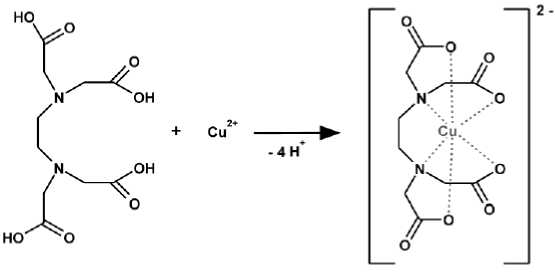
Copper(II)-Ion can form a stable [CuC 10 H 12 N 2 O 8 ]2-- or [CuC 14 H 32 N 2 O 4 ]2+-complex (Scheme 2 & 5) together with ethylene diamine tetra acetic acid (C 10 H 16 N 2 O 8 ) or with N,N,N`,N`-tetrakis(2-hydroxypropyl)-ethylene diamine
(C 14 H 32 N 2 O 4 ) tetra-ions [38]. The EDTA-anion is also available for a complex formation, besides the four carboxyl groups (-COOH) also two free electron pairs of the nitrogen atom and can be attached 6-times to the copper cations (Scheme 2).
Scheme 2 Formation of a stable [CuC 10 H 12 N 2 O 8 ]2-- complex.
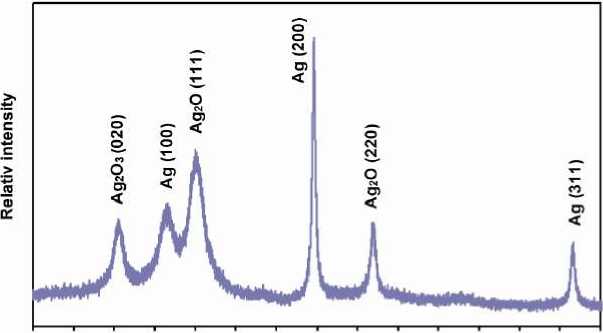
XRD pattern of the silver and silver oxide nanoparticles have been presented in Figure 6. The two-theta peaks of Ag 2 O 3 at 22° can be assigned to their characteristics (020) indices [39]. Two obvious peaks at 31° and 52° correspond to
-
(111) and (220) planes of Ag 2 O [40, 41]. The peaks at 27°, 44° and 77° can be assigned to (100), (200) and (311) reflections, respectively, of the face-centered cubic lattice of Ag (JCPDS date 04-0783) [10, 12].
10 15 20 25 30 35 40 45 50 55 60 65 70 75 80
2Ө (degree)
Figure 6 - XRD spectra of the silver and silver oxide nanoparticles on metalized PET-continuous filament yarn
The XRD pattern (Figure 7) shows strong diffraction peaks at 44°, 51° and 77° pertaining to cubic copper Cu (111), (200) and (220) metal crystals (JCPDS 85-1326) and the diffraction peaks at 59° relating to the formation of CuO (202) crystalline phases [4, 42]. It should be
noted that no peaks related to the cuprous oxide [Cu 2 O and Cu(OH) 2 ] phase were observed in the XRD spectra. This means that our synthesized samples substantially contained CuO and/or Cu phases for the nanoparticles.
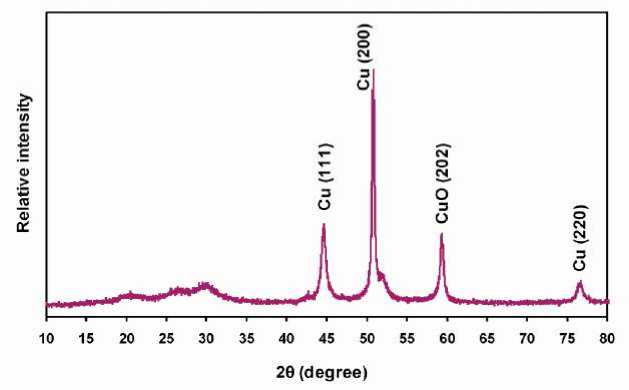
Figure 7 - XRD patterns of the copper and copper oxide nanostructures on metalized PET-continuous filament yarn
By taking the energy-dispersive x-ray spectroscopy (EDX) analysis into consideration, it can be seen that the percentage of oxygen increased and the percentage of carbon decreased after the alkaline pre-treatment (Table 1). The increase of the oxygen percentage can be ex- plained because the ester groups (-COO-) of the polyester which was transferred by an alkaline hydrolysis into carboxyl groups (-COOH) and hydroxyl groups (-OH). The pre-silvered test sample had a silver atom percentage of 0.52% (Table 1).
Table 1 - EDX-analysis of original, pre-treated and pre-silvered PET continuous filament yarn
|
Element |
original |
pre-treated |
pre-silvered |
||
|
Atom (%) |
Element (wt. %) |
Atom (%) Element (wt. %) |
Atom (%) |
Element (wt. %) |
|
|
C |
91.61 |
87.92 |
83.89 79.73 |
78.64 |
73.35 |
|
O |
8.39 |
12.08 |
16.11 20.27 |
11.54 |
13.16 |
|
N |
- |
- |
- - |
9.3 |
8.04 |
|
Ag |
- |
- |
- - |
0.52 |
5.45 |
After the second silvering process, an increase of the silver atom percentage (12.78 %) could be observed on the fiber surface using EDX-analysis (Table 2). An increase of the copper percentage (77.40 % wt.) in comparison to the silver percentage (55.82 % wt.) took place after the coppering process.
Table 2 -EDX-analysis of silvered and coppered PET continuous filament yarn
|
Element |
silvered coppered Atom (%) Element (wt. %) Atom (%) Element (wt. %) |
|
C O N Ag Cu |
75.14 36.54 47.88 17.34 9.77 6.33 4.22 2.04 2.31 1.31 0.00 0.00 12.78 55.82 7.51 3.23 - - 40.39 77.40 |
Metallization of Textile Fabrics. The newly deveoped wet-chemical one-bath method is not only used for the silvering of yarn but also for the silvering of textile fabrics. The optical properties of the pre-silvered, silvered and coppered PET warp knit fabric (Figure 8) are similar to yarn (Figure 2).
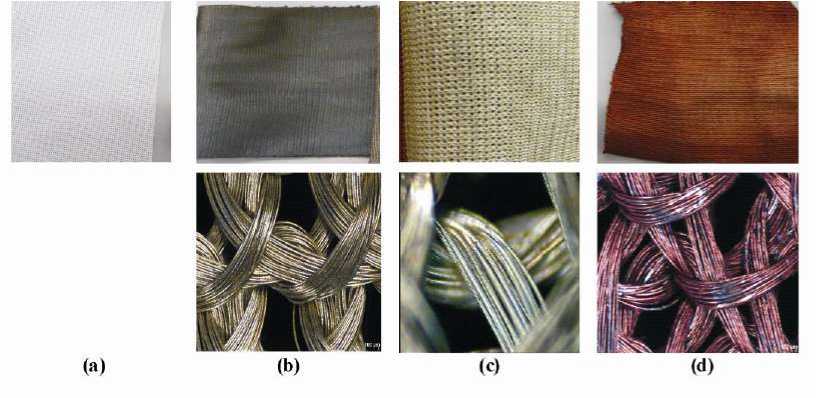
Figure 8 (a) Original, (b) pre-silvered, (c) silvered and (d) coppered PET warp knit fabric
The SM of original PET warp knit fabric and the pre-treated with sodium hydroxide solu- tion is identical as yarn and illustrated in Figure 9.
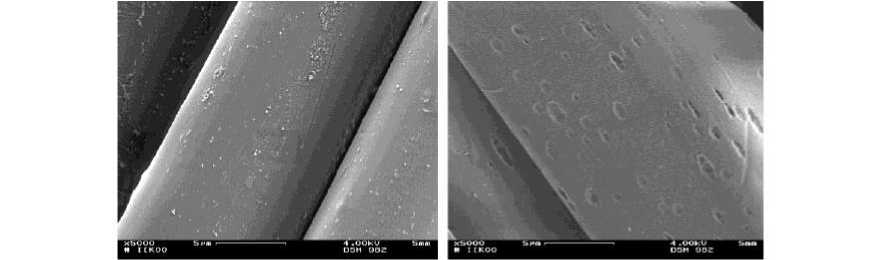
Figure 9 - SEM-images (x5000) of (left) original and (right) the one pre-treated with 0.5 N NaOH solution PET warp knit fabric
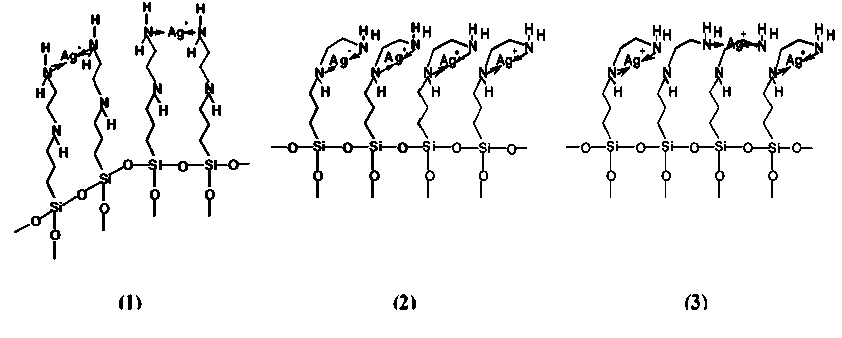
The pre-silvering of the activated warp knit fabric took place in an [Ag(C8H22N2O3Si)n)]+-complex solution. The N-(2-aminoethyl)-3-aminopropylentrimethoxysilane (C8H22N2O3Si) has two amino groups in its chain (Scheme 3). A silver diamine complex could be formed with a primary amino group of two silane chains each (1), with a primary and a secondary amino group of a silane chain (2) or alternated (3) [43].
Scheme 3 Complex formation possibilities of silver ions with N-(2-aminoethyl)-3-aminopropylentrimethoxy-silane (C 8 H 22 N 2 O 3 Si).
After the pre-silvering, an incompletely covered silver layer was formed on the PET-fiber surface (Figure 10a). A completely covered silver layer made of evenly distributed spherical silver particles could be determined after the second silvering process using the SEM-images (Figure
10b). The coppering of the pre-silvered fabric occurred in a [CuC 10 H 12 N 2 O 8 ]2+-complex solution. After the wet-chemical coppering, the copper layer increased on the pre-silvered fiber surface and the fiber surface was fully covered with a copper coating (Figure 10c).

Figure 10 -SEM-images (x20.000 and x50.000) of (a) pre-silvered, (b) silvered and (c) coppered PET warp knit fabric
The EDX-analysis showed that the oxygen percentage increased and the carbon percentage decreased after the alkaline pre-treatment (Table
-
3). The atomic Ag percentage of the pre-silvered PET warp knit fabric using the one-bath silvering method amounts to 24.28% (wt.).
Table 3 - EDX-analysis of original, pre-treated and pre-silvered PET warp knit fabric
|
Element |
original |
pre-treated |
pre- |
silvered |
|
|
Atom (%) |
Element (wt. %) |
Atom (%) Element (wt. %) |
Atom (%) |
Element (wt. %) |
|
|
C |
90.61 |
87.87 |
84.76 81.84 |
69.88 |
51.01 |
|
O |
9.39 |
12.13 |
15.24 18.16 |
12.65 |
12.42 |
|
N |
- |
- |
- - |
9.07 |
10.09 |
|
Si |
- |
- |
- - |
2.70 |
2.20 |
|
Ag |
- |
- |
- - |
5.70 |
24.28 |
After the second silvering process, the silver percentage increased from 24.28% (wt.) to 54.74% (wt.) (Table 4). The Cu percentage of the coppered PET warp knit fabric using the bath exhaustion method was 63.80% (wt.).
Table 4 - EDX-analysis of silvered and coppered PET warp knit fabric
|
Element |
silvered coppered Atom (%) Element (wt. %) Atom (%) Element (wt. %) |
|
C O N Si Ag Cu |
75.29 38.94 66.56 30.44 9.64 5.88 4.28 2.48 1.78 0.39 0.00 0.00 0.08 0.05 0.00 0.00 13.21 54.74 2.71 3.28 - - 26.45 63.80 |
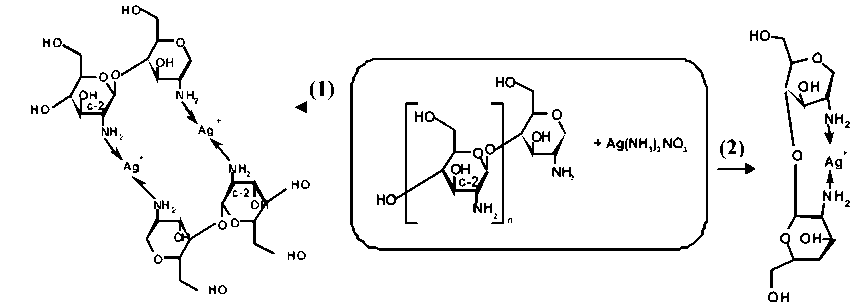
Metallization of Spacer Textiles. The developed one-bath method is flexibly applicable and has been used for the silvering of spacer fabrics (Figure 11a). Spacer fabrics consist of three “layers” – two covering layers and a spacer layer. The covering layers were produced with PET multi filament yarn (cover yarn) and the spacer layer made of PET mono filament yarn (pile yarn). The pre-silvering of the pretreated spacer fabric was carried out in a silver chitosan solution. Chitosan (CTS) is a linear biopolymer on the basis of polysaccharide, consists of two monosaccharide-blocks: N-acetyl-D-glucosamine and D-glucosamine, which bonded by β(1→4) glycoside [44]. „The chemistry of the chitosan is characterized by its nu-cleophil, primary amino groups at the C-2-atom of the glucan-unit (Scheme 4). At this point, many chemical functionalizations build“ [4547]. It is possible to bond the CTS-chains using the NH2-groups by complexing the silver amongst itself (Scheme 4, 1). Under the coordination of adjacent NH2-groups of the same chain, the silver forms a silver diamine complex as can be seen in Scheme 4 (2).
Scheme 4 Complex formation possibilities of silver ions with chitosan.
Through the wet-chemical pre-silvering, it was possible to modify the silver coating on an inert multi filament and mono filament PET fiber surface (Figure 11b). After the second silvering and the wet-chemical coppering, spacer fabric made of even the thickest PET mono filament was successfully metallized. Where, both the pile yarn (mono filament yarn, Figure 11c) and the cover yarn (multi filament yarn, Figure 11d) were fully metallized.
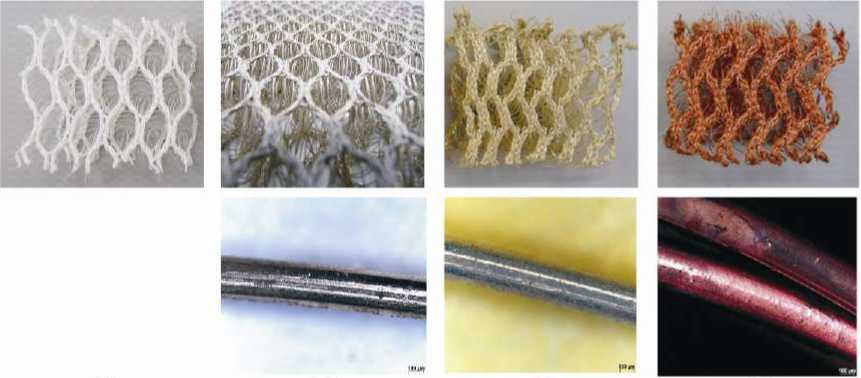
(a) (b) (c) (d)
Figure 11 (a) Original, (b) pre-metallized, (c) silvered and (d) coppered PET spacer fabric
The challenge for the silvering and coppering was the mono filament yarn (pile yarn) itself, because it has a very smooth and hydrophobic surface (Figure 12, left). The SM was analyzed by means of SEM in order to evaluate the pretreatment with sodium hydroxide solution in respect to their effects on the homogeneity of the fiber surface. During these analyses, a rough, clean mono filament fiber surface (Figure 12, right) was characterised after the pre-treatment with sodium
hydroxide
solution.
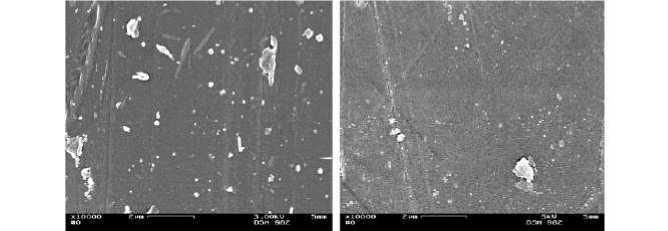
Figure 12 -SEM-images (x10.000) of (left) original and (right) the one pre-treated with NaOH solution mono filament yarn made of spacer fabric
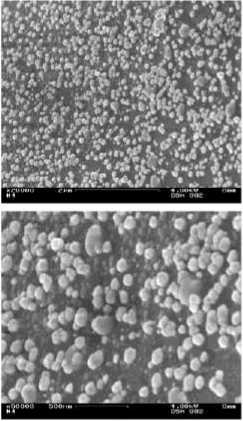
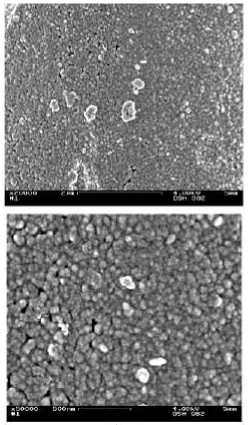
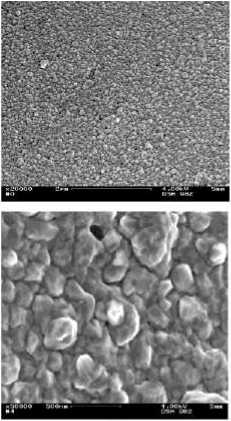
Figure 13 - SEM-images (x20.000 and x50.000) of (a) pre-silvered, (b) silvered and (c) coppered multi filament yarn from spacer fabric
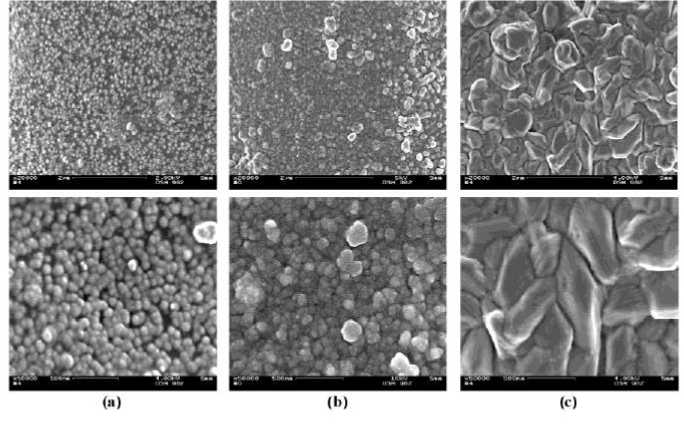
Figure 14 - SEM-images (x20.000 and x50.000) of (a) pre-silvered, (b) silvered and (c) coppered mono filament yarn from spacer fabric
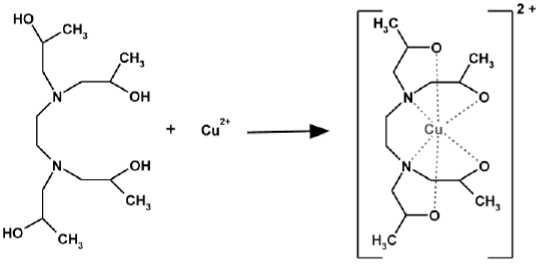
Copper(II)-cation can form [CuC14H32N2O4]2+-complex (Scheme 5) together with four oxygen atoms and two nitrogen atoms of the N,N,N`,N`-tetrakis(2-hydroxypropyl)-ethylene diamine.
Scheme 5 Formation of a stable [CuC 14 H 32 N 2 O 4 ]2+- complex.
The carbon-, oxygen-, nitrogen- and silver percentages are semi-quantitatively determined using the EDX-analysis (Table 5). After the pre- silvering process, the silver percentage was 14.08 % (wt.) for multi filament yarn and 24.41 % (wt.) for mono filament yarn.
Table 5 - EDX-analysis of untreated, pre-treated and pre-silvered PET spacer fabrics
|
Spacer fabric |
Element |
original |
pre-treated |
pre-silvered |
|||
|
Atom (%) |
Element (wt. %) |
Atom (%) |
Element (wt. %) |
Atom (%) |
Element (wt. %) |
||
|
Multi filament |
C |
83.76 |
79.54 |
82.52 |
76.68 |
76.20 |
65.68 |
|
yarn from spacer |
O |
16.24 |
20.46 |
17.48 |
23.32 |
9.39 |
10.07 |
|
fabric |
N |
- |
- |
- |
- |
12.10 |
10.17 |
|
Ag |
- |
- |
- |
- |
2.31 |
14.08 |
|
|
Mono filament |
C |
83.57 |
80.73 |
82.41 |
77.86 |
80.18 |
59.84 |
|
yarn from spacer |
O |
16.43 |
19.27 |
17.59 |
22.14 |
13.54 |
13.46 |
|
fabric |
N |
- |
- |
- |
- |
2.64 |
2.30 |
|
Ag |
- |
- |
- |
- |
3.64 |
24.41 |
|
After the second silvering process, the silver percentage of the multi filament yarn (42.88 %, wt.) and the mono filament yarn (59.21 %, wt.) of PET fiber surface increased again (Table 6). The carbon and oxygen percentage decreased after the coppering on the fiber surface and the highest copper percentage was indicated for the multi filament yarn PET fiber surface 83.15 % (wt.) using the EDX.
Table 6 - EDX-analysis of silvered and coppered PET spacer fabric
|
Filament yarn from spacer fabric |
Element |
silvered |
coppered |
||
|
Atom (%) |
Element (wt. %) |
Atom (%) |
Element (wt. %) |
||
|
Multi filament yarn |
C |
76.60 |
46.7 |
44.91 |
14.41 |
|
O |
11.70 |
9.30 |
2.25 |
0.96 |
|
|
N |
1.60 |
1.12 |
0.00 |
0.00 |
|
|
Ag |
10.10 |
42.88 |
3.86 |
1.47 |
|
|
Cu |
- |
- |
48.98 |
83.15 |
|
|
Mono filament yarn |
C |
72.40 |
33.09 |
70.52 |
38.82 |
|
O |
8.90 |
5.40 |
5.97 |
4.38 |
|
|
N |
4.25 |
2.30 |
0.00 |
0.00 |
|
|
Ag |
14.45 |
59.21 |
5.15 |
3.32 |
|
|
Cu |
- |
- |
18.36 |
53.48 |
|
By increasing the treatment temperature of 400°C to 600°C in an N2 (80%)-H2 (20%) can result higher chemical stability of the reduced copper nanoparticles in the silica thin film. 42 The silvered and coppered PET textile materials treatment by 400 °C is impossible, since a melting temperature of PET is from 250 °C to 270 °C. For this reason, the metalized PET textile materials were thermally fixed at 200 °C for 2 min. Deposited Ag- and Cu-layer shrinks, compacts and cures due to the vaporization of the coating and solvents, on-going hydrolysis and condensation reactions, the silver and copper particles diffuse from the surface into the substrate (PET). By decreasing the water content of the metal layer leads to increase the density of the metal layer.
EDX analysis / the percentage of the elements such as carbon (C), oxygen (O), nitrogen (N), silicon (Si), silver (Ag) and copper (Cu) was semi-quantitatively determined with the point analysis. Gravimetric analysis was carried out to examine realistic “global” values of silver and copper on surface of the textiles. The amount of matting agent of the original PET was 0.3 %. The results of AAS analysis revealed that higher amount of silver on the silvered textile materials was found compared to pre-silvered textile materials (Table 7).
Table 7 - The concentration of silver and copper on surface of the textiles
|
PET textile materials |
Metal Yarn Knit fabric 3D spacer fabric The concentration of Ag and Cu on surface of the textiles, (%) |
|
Pre-silvered silvered coppered |
Ag 1.26 1.75 1.54 Ag 5.84 6.54 6.26 Ag 5.23 6.55 6.28 Cu 1.41 1.57 1.42 |
Adhesion of the Silver and Copper Layer. Metalized textiles are often applied in different fields such as the medical one, in safety and in construction engineering, in water technology, in the clothing and automotive industry, in which they are exposed to mechanical, thermal or wet-chemical loads. In order to retain the functional characteristics of the tex- tiles for the entire working life, the adhesion stability of the silver and copper layer on the fiber surface is an essential quality characteristic. In the textile industry, however, often insufficient adhesion and wash fastness of metallized textiles creates the main problem for their use. Therefore, in the framework of this paper, silvered and coppered PET-substrates underwent several washing cycles to analyze their usability characteristics.

Figure 15 - Colour changes of the liquor, the silvered and attached (PA and WO) textile material after the first, fifth and tenth wash cycles
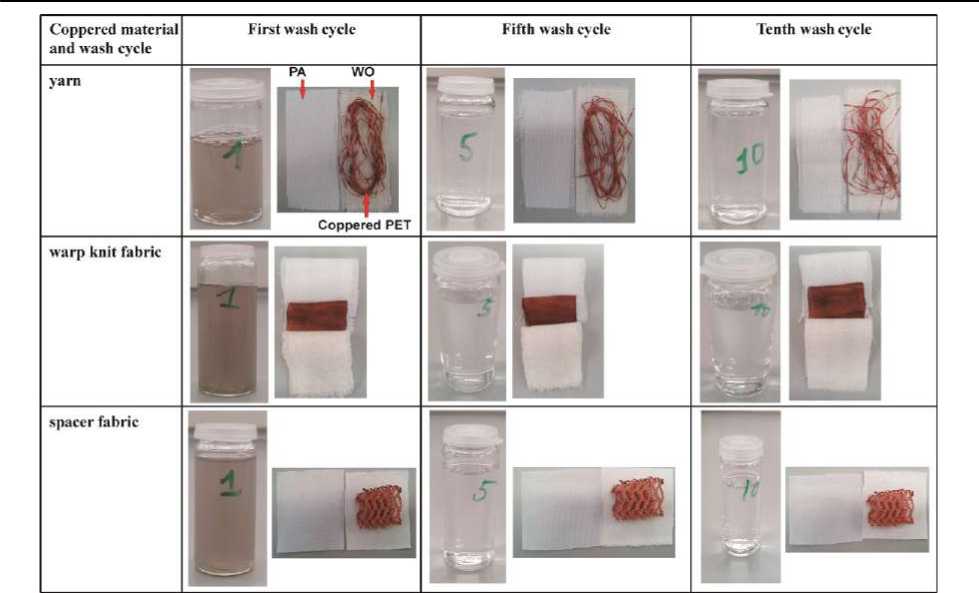
Figure 16 - Colour changes of the liquor, the coppered and attached (PA and WO) textile material after the first, fifth and tenth wash cycles
Conclusions
The key of the realization for the silvering and coppering of textile material made of polyester was the wet-chemical one-bath method with silver diamine complex-containing solution and with EDTA- and EDTP-copper-containing solution. All complexes are stable and suitable for the silvering and coppering of polyester yarn, warp knit fabric and spacer fabric. After the successful implementation of the developed entire concept, different types of silvered and coppered polyester textile material are subject to morphological analyses. A completely covered silver and copper layer was achieved on the polyester fiber surface. During the pre-silvering, silver cations was complexed with aliphatic tetraethylenpentamine, with long-chained N-(2-aminoethyl)-3- aminopropylentrimeth-oxysilane and with chitosan. While the coppering, the copper(II)-ion with ethylene diamine tetra acetic acid (EDTA) or with N,N,N`,N`-tetrakis(2-hydroxypropyl)-ethylene diamine (EDTP) tetra-ions form a stable Cu(II)EDTA- or Cu(II)EDTP-complex. This formed complex was modified wet-chemically using the one-bath method at 35°C±2°C on a chemically-inert PET fiber surface. The advantages of the newly developed one-bath method are lower energy consumption, low chemical use and the usability of the newly developed method for all polymers.
Furthermore, the adhesion and wash fastness of the silver and copper layer were analyzed, which showed superior results. At the end, it can be confirmed that the inert polyester can be wet-chemically silvered and coppered in different forms (yarn, warp knit fabric, spacer fabric) using the newly developed one-bath method. Further applications for electrical and heat conductivity, anti-microbial and fungicidal effects of structured textiles can be opened up. This is especially advantageous for the application in sensor technology, electronics, the automotive industry, the security field, the medical field and in water technology, where synthetic polymers such as polyester are increasingly used.
Additionally, the analyses of silvered and coppered textile material for the characterization of the antimicrobial effect, the electrical conductivity, the electromagnetic shielding and the tensile strength are subject to future works.
ACKNOWLEDGEMENT
We would like to thank the Federal Ministry of Education and Research for the financial support of the funded projects [BMBF 033R036B: „Silver and Copper Metalized Textile Material Made of Polyester for the Re- source-Efficient Application for the Elimination of Biological Contamination in Water Sys-tems“].
Список литературы Silvering and coppering of chemically inert textile materials by means of wet-chemical process
- Akhavan, O. Lasting antibacterial activities of Ag-TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation // J. Coll. And Int. Sci. – 2009. – 336. –PP. 117-124.
- Esteban-Tejeda, L., Malpartida, F., Esteban-Cubillo, A., Pecharromán, C., Moya, JS. Antibacterial and antifungal activity of a soda-lima glass containing copper nanoparticles. // Nanotech. – 2009. – 20. – P. 505701.
- Perelshtein, I., Applerot, G., Perkas, N., Wehrschuetz-Sigl, E., Hasmann, A., Guebitz, G., Gedanken, A. CuO-cotton nanocomposite: Formation, morphology, and antibacterial activity. // Surf. & Coat. Technol. – 2009. – 204. – PP. 54-57.
- Akhavan, O., Azimirad, R., Safa, S., Hasani, E. CuO/Cu(OH)2 hierarchical nanostructures as bactericidal photocatalysts. // J. Mater. Chem. – 2011. – 21. – pp. 9634-9640.
- Akhavan, O., Ghaderi, E. Copper oxide nanoflakes as highly sensitive and fast response selfsterilizing biosensors. // J. Mater. Chem. – 2011. – 21. – pp. 12935-12940.
- Akhavan, O., Ghaderi, E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. // Surf. & Coat. Technol. – 2010. – 205. – pp. 219-223.
- Heinlaan, M., Ivask, A., Blinova, I., Dubourguier, H.Ch., Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. // Chemosphere. – 2008. – 71. – pp. 1308-1316.
- Paschoalino, M., Guedes, NC., Jardim, W., Mielczarski, E., Mielczarski, JA., Bowen, P., Kiwi, J. Inactivation of E. coli mediated by high surface area CuO accelerated by light irradiation >360 nm. // J. Photochem. Photobiol., A: Chem. – 2008. – 199. – pp. 105-111.
- Liu, Y., Wang, X., Yang, F., Yang, X. Excellent antimicobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. // Micropor. Mesopor. Mater. – 2008. – 114. – pp. 431-439.
- Akhavan, O., Ghaderi, E. Capping antibacterial Ag nanorods aligned on Ti interlayer by mesoporous TiO2 layer. // Surf. & Coat. Technol. – 2009. – 203. – pp. 3123-3128.
- Kawashita, M., Tsuneyama, S., Miyaji, F., Kokubo, K., Kozuka, H., Yamamoto, K. Antibacterial silver-containing silica glass prepared by sol-gel method. // Biomater. – 2000. – 21. – 393-398.
- Akhavan, O., Ghaderi, E. Bactericidal effects of Ag nanoparticles immobilized on surface of SiO2 thin film with high concentration. // Current App. Phys. – 2009. – 9. – pp. 1381-1385.
- Akhavan, O., Abdolahad, M., Abdi, Y., Mohajerzadeh, S. Silver nanoparticles within vertically aligned multi-wall carbon nanotubes with open tips for antibacterial purposes. // J. Mater. Chem. – 2011. – 21. – pp. 387-393.
- Akhavan, O., Abdolahad, M., Asadi, R. Storage of Ag nanoparticles in pore-arrays of SU-8 matrix for antibacterial applications. // J. Phys. D: Appl. Phys. – 2009. – 42. – pp. 135416-135423.
- Dietzel, Y., Przyborowski, W., Nocke, G. Veredlung von textilen Flächengebilden mittels PVD-Technologien. // Research report, Project Nr.4- 755-70-5150-98/1; 4-7531.50-025150/01/1, – 2001.
- Benelmekki, M., Torrell, M., Xuriguera, E., Vaz, F., Teixeira, V. Structure and properties of silver clusters implanted in PET by PVD sputtering for Active Packaging Applications. // J. Nano Research. – 2012. – 18-19. – pp. 105-116.
- Gimpel, S., Möhring, U., Müller, H., Neudeck, A. The galvanic and electrochemical modification of textiles. // Narr. Fab. and Braid. Ind. – 2003. – 40. – pp. 115-120.
- Li, L., Dan, Y., Wang, L., Wang, W. Electroless silver plating on the PET fabrics modified with 3-mercaptopropyltriethoxysilane. // J. App. Poly. Sci. – 2012. – 3. – pp. 1912-1918.
- Zhang, H. Silver plating on hollow glass microsphere and coating finishing of PET/cotton fabric. // J. Ind. Text. – 2012. – 42(3). – pp. 283-296.
- Körner, E. Plasma treated Ag nanocomposite coating for producing antibacterial and cytocompatible textiles. // Internat. Techtextil Symp. – 2011. – pp. 1-25.
- Wolf, G. D., Giesecke, H. Chemisches Versilberungsbad. // German Patent DE 34 19 755 A1. – 1985.
- Ilić, V., Śaponjić, Z., Vodnik, V., Molina, R., Dimitrijević, S. et al. Antifungal efficiency of corona pretreated polyester and polyamide fabrics loaded with Ag nanoparticles. // J. Mater. Sci. – 2009. – 44. – pp. 3983-3990.
- Onsuratoom, S., Rujiravanit, R., Sreethawong, T., Tokura, S., Chavadej, S. Silver Loading on DBD Plasma-Modified Woven PET Surface for Antimicrobial Property Improvement. // Plasma Chem. Plasma Process. – 2010. – 30. – pp. 191-206.
- Chen, Ch.Ch., Wang, Ch.Ch., Yeh, JT. Improvement of Odor Elimination and Anti-bacterial Activity of Polyester Fabrics Finished with Composite Emulsions of Nanometer Titanium Dioxidesilver Particles-water-borne Polyurethane. // Tex. Resear. J. – 2009. – 80(4). – pp. 291-300.
- Jiang, S., Newton E., Marcus Yuen, Ch.W., Kan, Ch.W. Application of Chemical Silver Plating on Polyester and Cotton Blended Fabric. // Tex. Resear. J. – 2007. – 77(2). – pp. 85-91.
- Alivisatos, A.P. The use of nanocrystals in biological detection. // Nature Biotechn. – 2004. – 22. – 47-52.
- Starke, E., Landgraf, J. Integrierte Sensornetzwerke in Faserverbundwerkstoffen. // 13th Symp. „Magnetoresistive Sensors and Magnetic Systems“ – 2006.
- Alexsandrowicz, A., Zimmermann, N. Einsatzmöglichkeiten technischer Textilien im Fahrzeuginnenraum. // 16th Aachener Kolloquium Fahrzeug-und Motorentechnik. – 2007.
- Lauterbach, Ch. SensFloor®- mehr als nur Boden. // Höhenkirchen-Siegertsbrunn Future-Shape GmbH, – 2008.
- Integration von Mikrosystemen zur Herstellung von multifunktionalen intelligenten Schutztextilien (MST4IT). // BMBF-Project Nr. 16SV3424. – 2008 – 2011.
- Hund, RD., Rossbach, V., Lück, C., Rödel, H., Militz, D., Kreysig, D. Textile Systeme zur Prävention und Eliminierung von biologischen Kontaminationen in Trinkwasser- und anderen flüssigkeitsführenden Systemen. // ADITC. – 2007.
- Hund, RD., Rossbach, V., Lück, C., Rödel, H., Militz, D., Kreysig, D. Silvered textile materials water-disinfiction. // 47. Man-made fibers congress Dornbirn (Austria). – 2008.
- Lu, Y. Electroless copper plating on 3-mercaptopropyltriethoxysilane modified PET fabric challenged by ultrasonic washing. // App. Surf. Sci. – 2009. – 255. – pp. 8430-8434.
- Zhu, Q., Sun, J., He, Ch., Zhang J., Wang, AQ. Influence of Plasma Treatment on the Electroless Deposition of Copper on Carbon Fibers. // J. Macromol. Sci. Part A. – 2006. – 43. – pp. 1853-1865.
- Yuen, CWM., Jang, SQ., Kann, CW., Tung, WS. Application of electroless nickel plating in textile design. // Textile Asia, – 2006. – 37(12). – pp. 32-36.
- Cho, J., Moon, J., Jeong, K., Cho, G. Application of PU-sealing into Cu/Ni electroless plated polyester fabrics for e-textiles. // Fibers & Polymers. – 2007. – 8. – pp. 330-334.
- Schwarz, A., Hakuzimana, J., Westbroek, Ph. et al. A study on the morphology of thin copper films on para-aramid yarns and their influence on the yarn`s electro-conductive and mechanical properties. // Tex. Resear. J. – 2012. – 82(15). – pp. 1587-1596.
- Gmelin, L. Handbuch der anorganischen Chemie, Kupfer Koordinationaverbindungen mit neutralen und innerkomplexbildenene Liganden. – 1966.
- Rahman, MM., Khan, Sh.B., Asiri, AM., Alamry, KA., Al-Youbi, AO. Detection of Nebivolol Drug Based on As-grown Un-doped Silver oxide Nanoparticles prepared by a Wet-Chemical Method. // Int. J. Electrochem. Sci. – 2013. – 8. – pp. 323-335.
- Hong-Liang, F., Xiao-Yong, G., Zeng- Yuan, Zh., Jiao-Min, M. Study on the Crystalline Structure and the Thermal Stability of Silver-oxide Films Deposited by Using Direct-current Magnetron Sputtering Methods. // J. Korean Physical Soc. – 2010. – 56. – pp. 1176-1179.
- Sullivan, KT., Wu, Ch., Piekiel, NW., Gaskell, K., Zachariah, MR. Synthesis and reactivity of nano-Ag2O as an oxidizer for energetic systems yielding antimicrobial products. // Combustion & Flame. – 2013. – 160. – pp. 438-446.
- Akhavan, O. Chemical durability of metallic copper nanoparticles in silica thin films synthesized by sol-gel. // J. Phys. D: Appl. Phys. – 2008. – 41. – pp. 235407-235415.
- Gmelin, L. Handbuch der anorganischen Chemie, Silber, Teil B 6, Komplexverbindungen mit neutralen und innerkomplexbildenden Liganden: Silber (I)-Komplex mit N- und O- haltigen Liganden, Silber(I)-Verbindungen: Komplexe mit Ammoniak. Komplexe mit Aminen. – 1975. ISBN 3-540-93 306-9.
- Wittke, R. H. Darstellung und Untersuchung funktionalisierter Polymeroberflächen, // Dissertation. – 2005.
- Knittel, D., Schollmeyer, E. Chitosan und seine Derivate für die Textilveredlung. // Textilveredlung. – 1998. – 33, – pp. 67-71.
- Park, JW., Park, MO., Park, KK. Mechanism of metal ion binding to chitosan in solution, Cooperative inter- and itramolecular. // Bulletin Korean Chem. Soc. – 1984.
- Micera, G., Deiana, S., Dessi, A., Decock, P., Dubois, B., Kozlowski, H. Copper and vanadium complex of Chitosan. // In R. A. A. – 1986. – 6. – pp. 90-92.


