Synthesis of nanoparticle of copper in the presence of polyvinylpyrrolidone and the study of their properties
Автор: Shaikhova Zh.E., Zhexenbay N., Tausarova B.R.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Естественные науки
Статья в выпуске: 2 (111), 2016 года.
Бесплатный доступ
This article presents the results of studies of synthesis of copper nanoparticles in an aqueous medium in the presence of PVP. The influence of the molar ratio of reactants, pH, temperature, the process of synthesizing nanoparticles of copper was studied. Obtained hydrosols examined spectrophotometrically in the wavelength range from 400 to 800 nm («JENWAY»). The Hydrosol on the basis of copper nanoparticles open up the possibility of using them to obtain various materials with antibacterial properties.
Polivinylpyrrolidone pvp, hydrazine, copper nanoparticles, surface plasmon resonance spr, cellulose materials, spectrophotometric method
Короткий адрес: https://sciup.org/140205079
IDR: 140205079 | УДК: 677.027.62
Текст научной статьи Synthesis of nanoparticle of copper in the presence of polyvinylpyrrolidone and the study of their properties
In the development of modern nanotechnology play a significant role study of metal nanoparticles [1]. This is due, primarily, a wide range of possibilities of practical application, in which specific properties are used both the nanoparticles, copper and their modified materials. Search agent provides antimicrobial action, they in different environments, including drinking water, soil, personal care, plastics, and medical devices, surgical instruments, plasters, bandages, surgical masks and respirators, clothing, medical personnel. Therefore, a further search of antimicrobial agents that are safe for human health, is important. A comparative study of the antimicrobial activity of nanoparticles of silver, copper, zinc and aluminum have shown that metals inhibit the growth of Escherichia coli , and a number of toxic elements in descending order the following: Cu> Ag> Zn> Al. The few works indicate the value of the physicochemical characteristics of the nanoparticles in the manifestation of the antibacterial properties [2].
Good prospects for the use of metal nanoparticles in other areas of technology and in biology and medicine. Possible applications of nanoparticles in the diagnosis and treatment of various (including cancer) diseases as well as in immunochemical methods of investigation have been actively studied in a new direction of experimental medicine, called "Nanomedicine". It is shown in particular that the copper nanoparticles can be used to produce various materials with antibacterial properties [3]. In the US, for the treatment of wounds, burns, venous ulcers, eczema, acne ointments are widely used brands Acticoat, Nucryst, composed of copper nanoparticles. The function of copper in the body is well understood, and it proved an absolute necessity in all phases of wound healing process. The observed antimicrobial activity of copper nanoparticles is the reason for their use as antimicrobial agents in the creation of antiseptic medical and technical materials, coatings, paints, etc. Copper nanoparticles have significant potential for use in the production of modern catalysts, lubricants and composite materials in the establishment of medical equipment etc.
The easiest and cheapest way for their preparation is the synthesis of nanoparticles (nanoparticle) by reducing a copper ion (II) in solution, since it does not require sophisticated technical performance and allows to control the size and morphology of the products obtained. The authors conducted many studies the synthesis of copper nanoparticles in non-aqueous or aqueous-organic media [4], the reduction in non-aqueous solvents is difficult to obtain a relatively concentrated metal colloids due to the low solubility and degree of dissociation of salts as a source, and a reducing agent, the electron transport complications etc. At the same time, the formation of aqueous solutions of nanoparticles of copper metal may be accompanied by uncontrolled oxidation process and the task of a reliable synthesis will not be solved.
The purpose of this paper is to provide a highly effective and low-toxic preparations based on copper nanoparticles, determining the optimal conditions for the synthesis of nanoparticles of copper the presence of high-molecular compounds, the effect of various factors: the concentration of the solution, the molar ratio of reagents, pH, temperature on the recovery process of copper ions, the method of deoxygenation of stock solutions etc., as well as a study of the products obtained by means of spectroscopy.
Among the methods of preparation of nanoparticles to form a large group of chemical synthesis based on the reduction of metal ions to the atoms in solution under conditions conducive to the subsequent aggregation of the atoms and ions to form nanoparticles.
Materials and Methods of Research
Synthesis of nanoparticles was performed by reducing copper aqueous copper sulfate solution. As the reducing agent, hydrazine sulfate was used, the reaction oxidized to nitrogen. The structure and size of the product to a large extent depends on the reaction conditions, the concentration of copper sulfate. Copper nanoparticles with various sizes can be obtained by increasing the reaction time [5].
Synthesis of nanoparticles was performed by the following procedure: to 5 ml of aqueous copper sulfate solution (II) (C = 0.01-0.05 mol / L), an equal volume of polyvinylpyrrolidone (PVP) (0.01-0.1 mol / l ), then 5 ml of hydrazine solution (C = 1 3 M) and the resulting solution was basified with ammonia to a definite value pH (9-11). The recovery process is carried out at a temperature of 40 ° C, 60 ° C, 80 ° C and direct contact with air in order to investigate the stability hydrosols obtained to oxidation. Upon heating the solution for 5 min becomes brown tinge, indicating the formation of copper nanoparticles. Obtained hydrosols examined spectrophotometrically in the wavelength range from 400 to 800 nm («JENWAY») to establish the stability of the obtained sol over time.
Recovery of copper ions hydrazine can be described by the following equation
2CuSO 4 + 4NH 3 + N 2 H 4 →2Cu + N 2 ↑+ 2(NH 4 ) 2 SO 4 , which implies that the process is carried out in an alkaline medium.
The presence of copper nanoparticles can be established by the presence of the optical spectra of the so-called maximum surface plasmon resonance (SPR) at a wavelength of 700-730 nm depending on the properties of the system. Based on the analysis of the spectra, namely the shape and position of the maximum intensity of the SPR picked synthesis conditions [6].
The optical spectra of the hydrosol containing metallic nanoparticles, characterized by the presence of so-called maxima of surface plasmon resonance (SPR) that appear when the frequency of the incident electromagnetic wave and the natural oscillations of the electrons in the nanoparticle. Type, intensity and position of SPR determined by the size, shape and degree of oxidation of nanoparticles. For spherical copper nanoparticles (2-10 nm in size) corresponds to the position of SPR 700-730 nm. By increasing the thickness of the oxide film on the surface of copper nanoparticles occurs relative increase in absorption in the wavelength range 700-750 nm. In our opinion, the difference between the maximum intensity values SPR (ISPR) and optical absorption in the "red" region of the spectrum at 760 nm (I760).
Results and Discussion
The optical spectra of the hydrosol containing metallic nanoparticles, characterized by the presence of so-called maxima of surface plasmon resonance (SPR) that appear when the frequency of the incident electromagnetic wave and the natural oscillations of the electrons in the nanoparticle. Type, intensity and position of SPR determined by the size, shape and degree of oxidation of nanoparticles. For spherical copper nanoparticles (2-10 nm in size) corresponds to the position of SPR 700-730 nm. By increasing the thickness of the oxide film on the surface of copper nanoparticles occurs relative increase in absorption in the wavelength range 700-750 nm. In our opinion, the difference between the maximum intensity values SPR (I SPR ) and optical absorption in the "red" region of the spectrum at 760 nm (I 760 )
b = I SPR - I 760 (2) will be described as the output of nanoparticles and their degree of oxidation. This option is selected to optimize the process of producing copper nanoparticles in this study(Fig. 1).
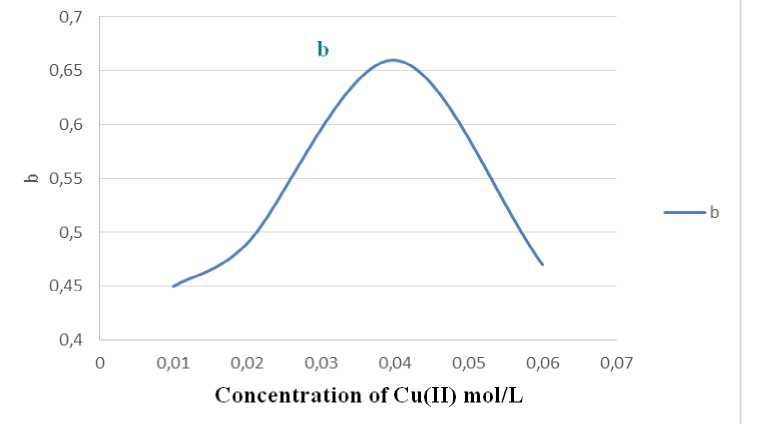
Figure 1 - Dependence on the concentration of b to Cu (II) mol / l
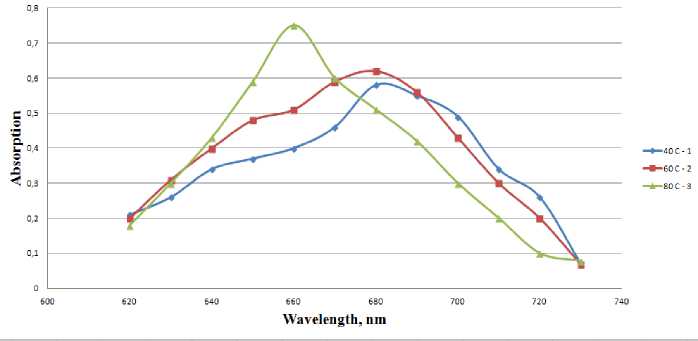
Figure 2 - Effect of temperature on the yield of the synthesis of nanoparticles. The change of the optical absorption spectra of copper-containing hydrosol at 1 – 400С, 2 – 60 0С, 3 - 80 0С
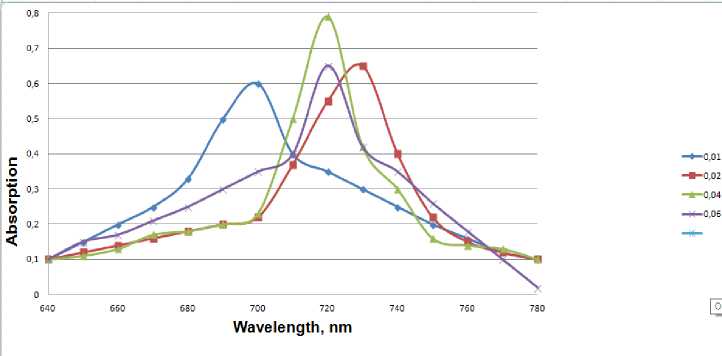
Figure 3 - Effect of sulfate concentration on copper nanoparticles yield: 1-1 * 10-2 M; 2-2 * 10-2 M; 3-4 * 10 -2M; 46 * 10-2 M;
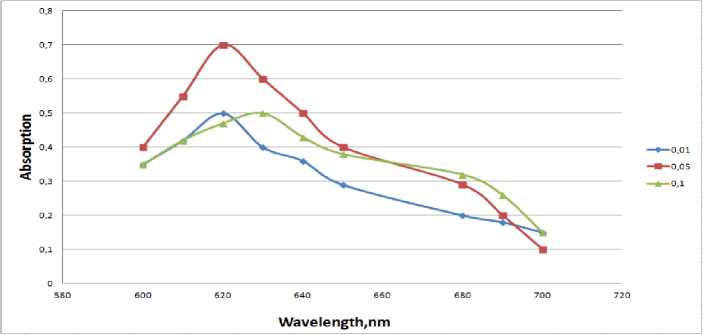
Figure 4 - Impact of the PVP concentration on the yield of nanoparticles: 1-1*10 -2М; 2-5*10 -2М; 3-1*10 -1М;
Effect of Temperature
Fig. 2 shows the release of nanoparticles from the process temperature (40 to 80 °C). It is seen that with increasing temperature, the relative density of the reconstituted product changes when 40 0С-0,58; 60 0С-0,62; 800 С-0,75.
Conclusion
The experiments were obtained from sedimentation stable salt concentration of copper nanoparticles about 0.05 mol / l. The optimal conditions for the synthesis of nanoparticles: pH 9-10, the initial concentration of ions of copper (II) - 0.04 mol /1 PVP - 0.05 mol /1 (Fig. 3. and Fig. 4), hydrazine - 1M. In the study of the sustainability of the colloids have found that the intensity of the maximum outage of C = 0.040.06 mol / l. is reduced to about 2 times, and stored sols sedimentation stability. Perhaps partial dissolution of nanoparticles under the action of atmospheric oxygen is not excluded as the formation of a surface film of copper oxide (I), which causes the same change in the spectra.
Список литературы Synthesis of nanoparticle of copper in the presence of polyvinylpyrrolidone and the study of their properties
- Olenin A.Yu. Getting the speaker volume and structure of the surface of metal nanoparticles in condensed media//Successes of chemistry, 2011.-№ 7, t.80. -P.635-662.
- Paltsev M.A. Nanotechnology in medicine and pharmacy//Remedium, 2008. -№9. -P. 6-12.
- Rakhmetova A.A., T.P. Alekseeva, Bogoslovskaya O.A., et al. Wound healing properties of copper nanoparticles according to their physico-chemical characteristics//Russian Nanotechnologies, 2010. -№ 3-4, V.5. -P. 102-107.
- Saikova S.V., Vorobyev S.A., Nikolaeva R.B., Mikhlin Yu.L. Determination of conditions for the formation of copper nanoparticles in the reduction of Cu2+ ions solutions of hydrazine hydrate//Journal of General Chemistry, 2010. -№ 6, T. 80. -P. 952-957.
- Kotelnikova N.E., Mikhailidi A.M. Modification linen materials copper particles // Chemistry of plant raw materials, 2009. - № 3. - P 43-48. Egorova E.M., Revina A.A. // Colloid Journal, 2002. - №3., T. 64. - P 334-345.


