The effectiveness of the waste water purification anion
Автор: Kurmanaliyev M.K., Sanazarova D.S., Ahmadi G.M.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Естественные науки
Статья в выпуске: 4 (113), 2016 года.
Бесплатный доступ
This article studies the influence of various factors on the sorption of chromium ions in the new anion exchangers Cybber AX 400 and Cybeer ALH 220. The degree of extraction of chromium ions from an aqueous solution on anion exchangers studied is 99,2%.
Anion exchangers cybber ax 400, cybeer alh 220, water waste, sorption, kinetic, ion exchange
Короткий адрес: https://sciup.org/140205091
IDR: 140205091 | УДК: 541.49.127
Текст научной статьи The effectiveness of the waste water purification anion
Wastewater electroplating, containing heavy plants contain elevated concentrations of ions of metal ions, cause enormous economic and chromium (VI), which exceed the maximum permissible limits (MRL for discharge into waters fishery 0.05 mg / l) is almost 200 times. To solve them use chemical, physico-chemical, electrochemical and biological methods. A special place among these methods take sorption methods of purification with a high degree of extraction of metal ions. Developing effective cost-effective process of sewage treatment from ions of chromium (VI) to the requirements of quality standards is relevant and environmentally important issue. The aim of this study is to evaluate the possibility of disposal of chromium-containing waste water by ion exchange adsorption.
Objects and Methods of the experiment
-
2.1. A study of the basic physicochemical properties of the synthesized ion exchangers [3]
-
2.2. A study of sorption properties of ion exchangers
Preparation of anion exchangers to work
Ion exchangers was poured in a beaker not less than five times volume of a saturated solution of sodium chloride and left for 24 hour for swelling, then were washed from the not reacting and secondary substances with the appropriate organic solvents (ethanol, dimethylformamide), water, repeatedly were transferred from salt Cl-form in hydroxyl 5% solution of sodium hydroxide and hydrochloric acid, then the anion exchangers in hydroxyl form washed with distilled water until neutral reaction of the filtrate till phenolphthalein. The synthesized ion exchangers kept the OH-form under the layer of water to avoid partial hydrolysis of functional groups in ionic salt forms.
Study of physicochemical properties of anion exchangers were carried out in HE-form.
For the determination of the static exchange capacity of the synthesized ion exchangers by the ions of metals used solutions of potassium dichromate (0,2; 0,5; 1,0; 2,0 g/l Cr).
The portion of the ion exchangers 0.1 g (based on dry ion exchanger), weighted with accuracy of 0.0002 g, poured 40ml of solution of studied salts. The solution contained 20 ml of salt, from 0 to 20 ml with an interval in 2 ml of 0.1 n sulfuric acid for determination of optimum pH value, water to a final volume of 40ml. After the keeping of equilibrium (24h) determined the equilibrium concentration of metal ions in solution by the method of atomic absorption spectroscopy in a flame air-acetylene spectrophotometer Perkin-Elmer-403. Sorption capacity (SEM, mg/g) of the resin was calculated by the formula:
СЕМ = ((С0-С)∙V)/P (2.1)
where C0 it is the initial concentration of metal ions in solution, g/l; C –equilibrium concentration of metal ions solution, g/l; V – volume of solution (40 ml); P – load of dry ion exchanger.
Sorption characteristics of anion exchangers was calculated as follows:
Кр = ((С0 – Comp.)∙V)/(Comp. ∙m) (2.2)
ε = (С0 – Comp.)/С0 ∙ 100 (2.3)
CE = ((С0 – Comp.)∙V)/m (2.4)
where Cu it is the coefficient of distribution, ml/g;
-
ε - the degree of extraction of iodine, %;
CE sorption capacity, mg/g;
-
m is the mass of dry adsorbent, g;
-
V – volume of the studied solution, ml;
C0 it is the concentration of element in the initial solution mg/l;
Ref. – the equilibrium (residual) concentration of extractable elements in the solution, mg/L.
Results and Discussion
We have studied the features of the interaction and the mechanism of ion exchange of chromium CR(VI) with the active groups of the anion exchange in the static and dynamic conditions.
Implementation of this method requires an available of the industry, non-deficient, easily regenerable sorbents. So, in this work, at first,we have to pay an attention to the selection of ions for the sorptivepreconcentration of chromium (VI) from sulfate solutions on different types of sorbents. To solve this problem we originally had taken a range of sorbents differing in their physical structure and functional groups. Characterization of the ion exchangers presented in the table 1 and 2 [2]:
Table 1 Characteristics of the anion exchanger Cybber-ALX 220
|
Parameter |
Themetric / value |
|
Functionalgroup |
Tertiaryamine |
|
The type of a matrix |
Macroporous |
|
Exchangecapacity, EQ/dm3 ≥ 1,45 |
≥ 1,45 |
|
Ionicform |
Thefreefoundation |
|
Moisturecontent, % |
50-60 |
|
Bulkdensity, g/cm3 |
0,65-0,71 |
|
Density, g/cm3 |
1,04-1,08 |
|
Granulessize, mm |
0,60-1,20 |
|
ThepHrange |
1-8 |
Table 2 Characteristics of anion exchanger Cybber-ALX 400
|
Parameter |
Themetric / value |
|
Functionalgroup |
Quaternaryammoniumbase |
|
The type of a matrix |
Gel |
|
Exchangecapacity, EQ/dm3 ≥ 1,45 |
1,3 |
|
Ionicform |
Basic |
|
Moisturecontent, % |
50-60 |
|
Bulkdensity, g/cm3 |
0,66-0,70 |
|
Density, g/cm3 |
1,10-1,12 |
|
Granulessize, mm |
0,56-1,10 |
|
ThepHrange |
1-14 |
It should be noted that all the ion exchangers were synthesized in the St. Petersburg JSC Synthesis and all of them in the new samples, not previously examined with sorption of chromium from solutions.
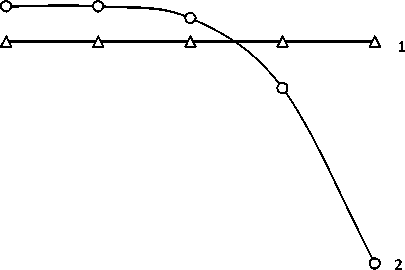
A significant influence on the sorption process has a pH. Adjusting the acidity can significantly increase the degree of extraction of metal sorption method, which is important when solving technological problems. Picture-1 presents the results of research of influence of pH on sorption capacity.
SС(mg/g) 300
0 "1----------------1----------------г
1-----------,-----------,-----------, рН
6 8 10 12
Picture-1 Effect of pH medium on the sorption capacity of anion exchangers: 1 - OH 400; 2 - АLX 220 .
From the picture 1 it is seen that pH does not affect the sorption ability of anion exchanger 400 AH. The maximum value of the exchange capacity of the sorbent ALX 220 is observed in acidic medium at pH = 2 – 6. At pH>7 there is a sharp decrease in the adsorption of chromium ion. This phenomenon can be explained by the change in the degree of dissociation of ionogenic groups of the sorbent. For the further studies on anion selected pH pH = 2 to 4, since the pH values of maximum sorption capacity of the sorbent.
Sorption capacity of the adsorbent and kinetic parameters are important indicators that determine the possibility of using this material for practical purposes. For the studying the kinetics of sorption of chromium ions (VI) sample of adsorbent weighing 0.1 g was placed in a strongly acidic solution of a metal salt with an initial concentration of chromium ions (VI) of 200
mg/DM (3,85 mmol/DM) and stirred it in the temperature up 293 K until equilibrium. The choice of a value for the concentration of chromium ions in the solution conditioned a significant sorption capacity of the adsorbent in relation to a test substance. The use of low concentrations does not allow for kinetic studies, as in this case that the process goes quickly. The initial concentration of chromium ions in model solutions selected 200 mg/dm3 (3,85mmol/DM) to study the sorption properties of the adsorbent in the conditions in which the concentration of chromium ions in the solution and 2-3 times higher than the maximum capacity of the adsorbent on this substance. To create a highly acidic environment used hydrochloric and sulfuric acid of different concentrations. The best indicators of sorption are observed when the using of sulfuric acid. The main kinetic dependence was the change in the magnitude of sorption in time: A=1(τ), where A it is the magnitude of sorption is reached to the time τ. Kinetic curves of the sorption process with the use of various anion exchangers shown in picture-2.
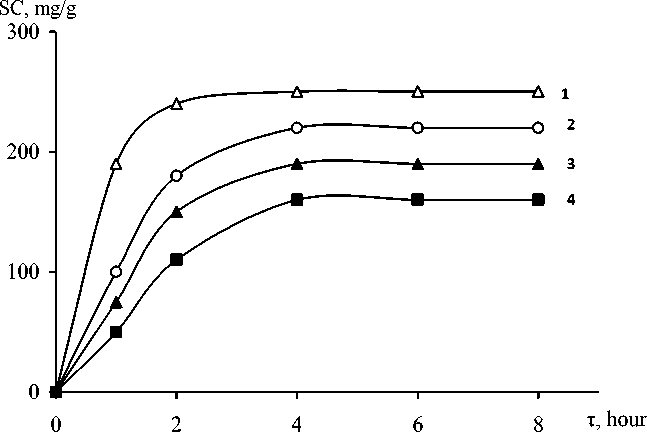
Picture-2 The Kinetic curves of sorption of chromium in the anion AH-400(3,4) and ALX-220(1,2). Initial solution concentration was 200 mg/l (1,3) and 100mg/litre (2,4). pH= 4, T=294К.
On the basis of the data obtained revealed that the sorption interaction in the system chromium-containing solution, the ion exchange proceeds rather intensively. The figure shows that the curves have a classic look and the balance of this system is reached to 8 g and the sorption process is substantially influenced by the nature of sorbent and the initial concentration of the investigated solute.
Calculations carried out on the basis of these data shows that the investigated ion exchangers have a maximum SOY by ions of chromium (VI) for ALX is 250 m g/g, and OH 210мг/g, this corresponds to the characteristics of these sorbents. The percentage of recoverability of chromium ions (VI) decreases with increase in the concentration of a stock solution of chromium salts.
Temperature is one of the important factors, which affecting the sorption equilibrium. Sorption processes are always accompanied by the decrease of free surface energy of the sorbent. So they must be exothermic processes, and with increasing temperature sorption should decrease. However, this assumption is undoubtedly only for physical adsorption. In the case of chemisorption, surface chemical reactions may be endothermic and exothermic. For substances, such as solubility increases with increasing temperature, at high temperatures one can expect certain conditions, the increase in sorption. The temperature selected for research, which determined by the temperature of the wastewater which varies in the range 20-40°C (293-313K). So, for the study is selected temperature as 299, 304 and 314К. To evaluate the effect of temperature on the sorption process solution was prepared with an initial concentration of chromium ions was 200 mg/dm3 (3,85 mmol/dm3). The acidity of the medium. 4. There is we took place in a solution sample anion exchanger OH ALX 400 or 200 weight of 0.12, was stirred until saturation of the adsorbent, in certain intervals was sampled to measure optical density. Next on the schedule graduivous determined the concentration of chromium ions (VI) in solution.
According to the data obtained adsorption isotherms of chromium ions (VI) at temperatures 294,304 and 314К (picture-3).
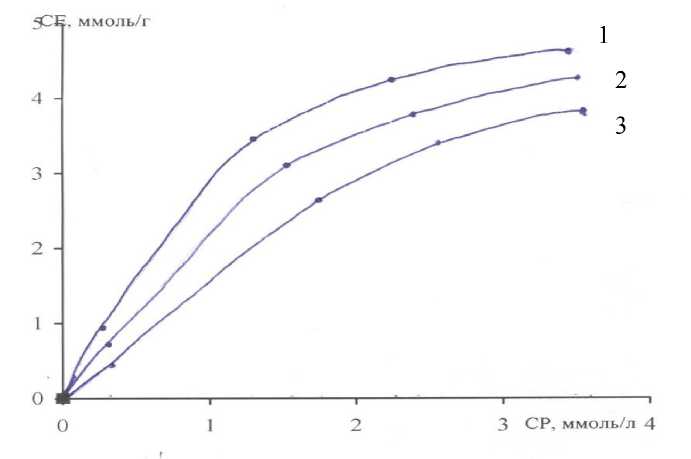
Picture-3 Isotherm of sorption of chromium ions by anion exchanger 400 AH at different temperatures sorption: (1) 314К, (2) 304К, (3) 294К.
The dependences shows that with increasing temperature the amount of adsorption increases. The maximum sorption is observed at 314К. The analysis of isotherms of sorption of chromium ions (VI), is presented in figure-3.3, shows that they belong to the isotherms of monomolecular adsorption. Curves monotonically approaches a certain limit value corresponding to the filling of the monolayer. Active centers on the surface of the adsorbent, increase its ion-exchange properties, or may be due to a significant sorption capacity of the anion exchanger in relation to ions of chromium.
Thus, the anion exchangers exhibit high investigated and kinetic sorption properties in relation to the chromium ion.
Conclusions
Studied sorption extraction of ions of chromium (VI) different types of new exchangers Cybber-AX 400 and Cybber-ALX 220 macroporous and gel structure.
Established the optimum sorption capacity of anion exchangers in static conditions: pH=4-6, adsorption of 6-8 hours, concentration of the stock solution 2 g/dm3, the temperature of the contact 294-304К. The sorption capacity of the investigated anion exchangers: ALX= 250mg/g; AH= 210мг/g.
For the full characteristics of anion exchangers explored sorption extraction of chromium in the dynamic mode. Found the full exchange capacity of the anion exchanger АLХ 220, which is 320мг/g.
Shown the efficiency of extracting ions of chromium (VI) from the model solutions of potassium dichromate anion exchangers new. Found that on their sorption properties, they surpass industrial anion exchanger the AV-17.
Список литературы The effectiveness of the waste water purification anion
- А.V. Novikov./Improving the quality of natural and waste water treatment/Novikov А.V., Zhenihov I.N. -Tver, 2006.-112p.
- Description anion exchangers Cybber . Access mode: http://www.szhk.ru/osnovnye-smoly-dlya-gidrometallurgii.
- N.G. Polyanskiy/Research methods of ion exchangers/Polyanskiy N.G., Gorbunov G.V., Polyanskaya N.L.-М.: Chemistry, 1976. -208 p.


