Ускоренное выздоровление. Актуальность, история, патофизиология
Автор: Воробьев Владимир Анатольевич, Белобородов В.А., Тухиев А.Р.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Организация урологической помощи
Статья в выпуске: 4 т.15, 2022 года.
Бесплатный доступ
Введение. Программа ускоренного выздоровления (ПУВ), хирургия ускоренной реабилитации (Fast Track surgery - FTS), а также ускоренное восстановление после операции (enhanced recovery аfter surgery/rapid recovery after surgery - ERAS) - мультимодальная и мультидисциплинарная стратегия лечения, предназначенная для сокращения сроков нетрудоспособности и улучшения качества оказания медицинской помощи. Данная программа включает подготовку на предоперационном этапе, использование минимально инвазивной техники выполнения хирургического вмешательства и активное ведение послеоперационного периода с целью уменьшения сроков стационарного лечения, времени реабилитации и максимально быстрого возвращения пациентов к обычной жизни. Одним из ключевых факторов успешности ПУВ является внедрение мультидисциплинарного взаимодействия на всех этапах обследования и лечения. Кратко охарактеризована история развития программ ускоренного выздоровления, актуальность разработки и применения оптимизированной стратегии лечения, описаны проблемы интеграции, мультидисциплинарности. Представлен обзор особенностей патофизиологии периоперационного стресса и практические выводы, обосновывающие разработку и применение элементов программы ускоренного выздоровления.
Протокол ускоренного выздоровления, ускоренное выздоровление, enhanced recovery а/ter surgery
Короткий адрес: https://sciup.org/142236645
IDR: 142236645 | DOI: 10.29188/2222-8543-2022-15-4-10-17
Текст научной статьи Ускоренное выздоровление. Актуальность, история, патофизиология
Программа ускоренного выздоровления – ПУВ (синонимы: хирургия ускоренной реабилитации – Fast Track surgery – FTS, ускоренное восстановление после операции – enhanced recovery аfter surgery/rapid recovery after surgery ERAS) – это мультимодальная и муль-тидисциплинарная стратегия лечения, предназначенная для сокращения сроков нетрудоспособности и улучшения качества оказания медицинской помощи. Данная программа включает подготовку на предоперационном этапе,использование минимально инвазивной техники выполнения хирургического вмешательства и активное ведение послеоперационного периода с целью уменьшения сроков стационарного лече-ния,времени реабилитации и максимально быстрого возвращения пациентов к обычной жизни.Одним из ключевых факторов успешности ПУВ является внедрение мультидисциплинарного взаимодействия на всех этапах обследования и лечения [1].
ИСТОРИЯ ВОПРОСА
В 1980-м году W.F. Finn опубликовал первую ста-тью,посвященную ускоренному выздоровлению при урологической патологии, работа выполнена в эксперименте и посвящена быстрому восстановлению крыс после острой почечной ишемии [2]. За последующие 15 лет большая часть научных изысканий в рамках стратегии была посвящена ишемическим повреждениям различных органов и лечению инфекционных агентов. В 1990-м году B.R. Birch и соавт. представили результаты применения флумазенила в рамках программы хирургии одного дня при хирургическом лечении урологических заболеваний. Применение антагониста бензодиазепиновых рецепторов позволило сократить сроки постнаркозного восстановления при таких урологических процедурах как уретротомия, инцизия шейки мочевого пузыря, вазэктомия и прочее, без увеличения риска развития осложнений [3].
Концептуальное понимание принципов ускоренного выздоровления сформулировано впервые R.M. Engelman и соавт. в 1994 г. на примере восстановления после перенесенного коронарного шунтирования [4].
Первым систематический подход в формировании программы ускоренного выздоровления применил H. Kehlet в 1995 году, опубликовавший несколько статей, посвященных различным аспектам стратегии [5, 6]. В том числе были представлены результаты клинического исследования программы ускоренного выздоровления после колоректальных вмешательств у 18 пациентов. Сформулированы выводы: сбалансированная анальгезия, ранняя мобилизация и пероральное послеоперационное питание способны сократить сроки вос- становления [7]. Шестью годами позднее совместно с D.W. Wilmore профессор H. Kehlet опубликовали окончательно сформулированную концепцию стратегии «fast track surgery» [8]. Представленные публикации дали старт разработке аналогичных программ в смежных хирургических специальностях, в том числе и в урологии.
В 1996, 1997 и 1999 годах опубликованы результаты применения программы ускоренного выздоровления при выполнении трансуретральной резекции и вапоризации предстательной железы [9-11]. Дальнейшее концептуальное изучение привело к формированию протокола, допускающего досуточное пребывание и раннее удаление мочевого катетера после трансуретральной резекции предстательной железы с сопоставимым риском развития осложнений [12].
В последующем понимание технологии Fast track было усложнено, и в 2000 году дан старт новой, более сложной стратегии ускоренного выздоровления после хирургического лечения (ERAS) [13]. С каждым годом отмечается все большее вовлечение медицинских работников в разработку и применение программ.
С момента формирования концепции ускоренного выздоровления при хирургических операциях и по настоящее время отмечается устойчивая тенденция к росту количества научных публикаций в рецензируемых журналах и в настоящее время опубликовано более 6000 научных статей (рис. 1). Однако количество работ по проблематике ПУВ представляется незначительным в сравнении с общей публикационной активностью (всего на PudMed с 1995 года зарегистрировано 4,086,984 публикаций по хирургии и урологии, из которых лишь 0,15% посвящены ПУВ).
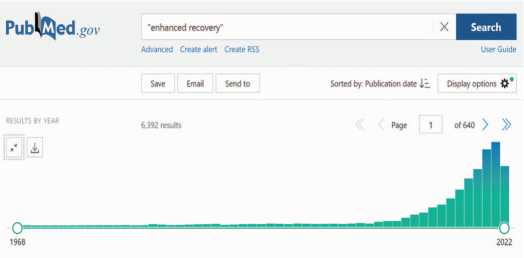
Рис. 1. Хронометрическая диаграмма результатов поиска по запросу «en-hanced recovery» в базе данных медицинских и биологических публикаций PubMed
Fig. 1. Chronometric diagram of search results for the query «enhanced recov-ery» in the English-language text database of medical and biological publications PubMed
АКТУАЛЬНОСТЬ ПРОБЛЕМЫ
Хирургическая операция – это преднамеренное повреждение человеческого тела, применяемое с целью лечения болезни или восстановления утраченных функ ций и тканей. По приблизительным данным ВОЗ, полу ченным на основании статистического анализа, экспериментальная и клиническая урология № 4 2022 ежегодно выполняется более 300 миллионов хирургических операций, каждая четвертая из которых оканчивается осложнением того или иного уровня. Также установлена прямая связь между количеством хирургических операций на душу населения и общей продолжительностью жизни в стране [14].
Развитие медицинских технологий привело к усложнению процесса лечения хирургических заболе-ваний.Разработаны и внедрены сложные эндовидео-скопические инструменты,камеры с высокой разрешающей способность и трехмерной визуализацией, методики дополненной реальности,применяются новые энергетические воздействия – все это повышает точность и уменьшает травматичность хирургических операций. Анестезиология также претерпела серьезные изменения: оптимизирован контроль боли, улучшено сохранение функции жизненно важных органов, уменьшены постнаркозные негативные явления,что позволяет максимально быстро возвращаться к нормальному состоянию после перенесенной анестезии. Содружественно изменились подходы к послеоперационному уходу и реабилитации в целом, сформировано понимание психологической подготовки и преабилитации.Все указанные меры направлены на уменьшение хирургического и анестезиологического стресса, замедляющего и нарушающего послеоперационное восстановление [13]. Таким образом сформировано понимание обязательного мультидисциплинар-ного взаимодействия в рамках реализации программы ускоренного выздоровления.
Помимо непосредственных организационных и технических мер огромное влияние на развитие медицины оказали информационные технологии.Доступ-ность информации практически в любой точке мира позволяет легче делиться новыми медицинскими зна-ниям,повышает общую квалификацию медицинских кадров и информированность пациентов.Однако описанные тенденции приводят к увеличению стоимости лечения. Спрос на качественные медицинские услуги, особенно хирургию, неуклонно растет. Возникает дуалистическое противостояние желания оказать качественную медицинскую помощь и необходимости экономить финансовые средства. Таким образом, любая оптимизация периоперационного периода,спо-собная сократить риски осложнений, сроки госпитализации и иным образом снизить стоимость,являются прямым благом для всех участников оказания помощи [13, 15, 16].
Внедрение инноваций в медицине сталкивается с очень серьезным сопротивлением и инерцией медицинского сообщества. Парадоксально что одни и те же люди (врачи и медсестры), использующие инновации в повседневной жизни, применяющие все более сложные гаджеты и регулярно их обновляющие, продолжают при оказании медицинской помощи руководствоваться принципами десяти- или пятнадцатилетней давности. Внедрение принципов ускоренного выздоровления при всей их доказанности и подтвержденной эффективно сти выполнено на незначительном уровне в рамках всей системы здравоохранения [13]. Одной из основ ных проблем при внедрении считается не технологиче ский дефицит, а сложности в восприятии и внедрении мультидисциплинарного взаимодействия, а также со противление администрации и медицинского персо нала.
Хирургическая операция – это всегда командная работа. Мультидисциплинарное взаимодействие со провождается усложнением медицинской науки. Чем более углубленной становится изолированная специ альность, тем сложнее специалисту установить взаимо действие с коллегами из других подразделений. Универсализм в медицине становиться редкостью, так как объем необходимого знания значительно превы шает возможности усредненного врача. Также большую роль носят индивидуальные предпочтения специали ста, который в силу собственного решения может иг норировать стандарты оказания помощи и использовать альтернативные методы лечения. И что не менее важно, установлено фактическое отсутствие времени и желания обучаться у большинства практикующих спе циалистов [13, 17].
ПАТОФИЗИОЛОГИЧЕСКОЕ ОБОСНОВАНИЕ
При разработке и внедрении программ ускорен ного выздоровления важно понимать, что разрабаты ваемые меры носят не только организационный характер, но и напрямую влияют на физиологию и патофи зиологию операционной травмы.
Хирургическая травма, боль, кровотечение, голо дание,ограничение мобильности – наиболее важные факторы, негативно влияющие на организм пациента. Их влияние носит не изолированный,а синергетиче ский характер, и способно значительно преумножаться по непредсказуемому сценарию. Исходом является ак тивация цепочки воспалительных реакций и симпати ческой нервной системы, что приводит к нарушению обмена инсулина. Как следствие, нарушается распре деление не только углеводов, но и липидов, и белков. Происходит централизация потребления глюкозы, снижение потребления ее на периферии и,как след ствие, развивается гипергликемия. Начинается разру шение гликогена, запускается протеолиз. Указанные нарушения обмена веществ особо важны для пациен тов с исходным нарушением обмена углеводов.
Обмен углеводов. Поддержание нормального уровня гликемии обеспечивается двумя основными механизмами:поглощение глюкозы тканями и ее вы работка печенью.Под воздействием хирургического стресса происходит высвобождение катехоламинов
(катехоламины, глюкагон, кортизол, гормон роста) и провоспалительных цитокинов (фактор некроза опу холи-альфа (TNF-α); интерлейкинов (IL-1, IL-6). Как следствие увеличивается выработка глюкозы и снижается ее потребление в тканях, возрастает уровень гликемии. Экспериментальным путем доказано, что уровень стрессовых реакций напрямую зависит от объема хирургической травмы. Малый объем травмы оказывает незначительное влияние на развитие гликемии [18]. Данный постулат представляется особенно важным в рамках программ ускоренного выздоровления.
При плановых лапаротомических и торакотоми-ческих операциях уровень глюкозы крови у недиабетических пациентов возрастает до 10 и 15 ммоль/л соответственно. При проведении лапароскопических операций уровень гликемии оказывается достоверно меньшим ( p <0,01), что свидетельствует о сниженном стрессовом ответе при меньшем повреждении именно скелетной мускулатуры [19].
Существенное влияние на гликемию оказывают и меры анестезиологической поддержки. Применение внутривенных анальгетиков (например, пропофола) не оказывает влияние на уровень глюкозы в крови. Наркотические опиатные препараты или нейроаксиальные методы обезболивания снижают гипергликемический ответ. Ингаляционные наркотические препараты и катехоламины, а также парентеральное питание усугубляют стрессовую гипергликемию [20].
Предоперационный стресс, как комплекс психогенных и патофизиологических процессов, также влияет на уровень гликемии. Каждый четвертый пациент без ранее установленного диагноза сахарного диабета в предоперационном периоде демонстрирует аномальный уровень гликемии натощак. Только у одного из десяти пациентов, страдающих сахарным диабетом, в предоперационном периоде был выявлен нормальный уровень глюкозы крови [21].
Таким образом, на основании множества исследований установлено, что основным патогенетическим механизмом хирургического стресса является именно снижение чувствительности к инсулину и нарушение обмена углеводов.
Первый вывод: применение 100 мл 5% раствора глюкозы приводит к двухкратному росту уровня гликемии в интраоперационном периоде. Это обусловлено негативным влиянием катехоламинов на эндогенную регуляцию секреции глюкозы. При отсутствии хирургического стресса вливание раствора глюкозы подавляет эндогенную ее секрецию, однако при стрессовом воздействии данный механизм не работает [22].
Второй вывод: каждому пациенту требуется контроль уровня гликемии в периоперационном периоде, особенно интраоперационно. Однако стандартное определение уровня гликемии лабораторией не позво- ляет выполнять оценку своевременно,а глюкометры или газоанализаторы крови не могут достичь нужного уровня достоверности результатов. В настоящее время проблема интраоперационного контроля гликемии остается не решенной [23, 24].
Формально считается, что гипергликемический ответ способствует лучшей сопротивляемости тканей стрессу, за счет лучшего обеспечения энергией. В пер вую очередь это необходимо клеткам крови, нейроци там и иммунным клеткам. Однако воздействие катехоламинов приводит к ингибированию инсулиннеза висимого мембранного транспорта глюкозы в мио карде и скелетной мускулатуре и экспрессии в клетках головного мозга и иммунных клетках. Так как обмен указанных клеток не регулируется инсулином, это при водит к их перегрузке глюкозой, развитию гликозили рования внутриклеточных белков,деактивации иммуноглобулинов, снижению хемотаксиса и фагоцитар ной активности нейтрофилов [25]. Итогом становится избыток супероксидных радикалов, митохондриальная дисфункция и апоптоз. Закономерным исходом стано вится ухудшение результатов хирургического лечения даже при небольшом возрастании гликемии [26]. Па циенты с гипергликемией натощак имеют в восемна дцать раз большие риски послеоперационной леталь-ности,продленной госпитализации и риски развития осложнений в сравнении с пациентами с нормальным уровнем глюкозы крови [27, 28].
Важно отметить, что гипергликемия способствует развитию послеоперационных, в том числе хрониче ских болей. А коррекция гликемии позволяет снизить послеоперационную болезненность и потребность в анальгетиках [25]. Также гипергликемия приводит к неврологическим расстройствам центральной нервной системы,таким как расстройство личности и сниже ние когнитивной функции [29]. В крупном когортном исследовании на более чем шестидесяти тысячах паци ентов установлено ( p <0,003), что периоперационная гипергликемия приводит к увеличению риска развития осложнений и общей однолетней смертности [30].
Инсулин выполняет несколько важных метаболи ческих (регулирование уровня глюкозы и ее потребле ния, стимулирование синтеза белка и ингибирование протеолиза) и не метаболических функций (сосу дорасширяющие, противовоспалительные, антиокси дантные,положительные инотропные и антифибрино-литические воздействия) [31].
Инсулинорезистентность – это любое состояние, сопровождающееся снижением отзывчивости (то есть изменение максимального ответа на инсулин при не изменной концентрации) или чувствительности к ин сулину (то есть влияние концентрации инсулина на достижение эффекта). Снижение чувствительности связано с изменение взаимодействия с рецепторами, а уменьшением ответа – с пострецепторным экспериментальная и клиническая урология № 4 2022 взаимодействием [32 ]. В рамках хирургического стресса развивается именно изменение чувствитель ности к инсулину. Это обусловлено высвобождением контринсулярных (и других контррегуляторных) гор монов, активирующих катаболизм, подавляющих вы работку инсулина и его периферическое действие [33]. Также в развитии инсулинорезистентности при нимают участие медиаторы воспаления, например интерлейкин 6 [34].
Количественно наиболее важный орган для по требления глюкозы в контексте развития инсулиноре зистентности – это скелетная мускулатура и миокард. Послеоперационная инсулинорезистентность дости гает максимального патофизиологического эффекта примерно через сутки после хирургической травмы и сохраняется до двух-трех недель. На длительность и выраженной резистентности к инсулину влияет объем (инвазивность) и продолжительность хирургической травмы, иммобилизация после операции, вид анесте зиологического пособия, кровопотеря, послеоперационное голодание, общее физическое состояние организма, меры преабилитации и реабилитации [35, 36].
Практический вывод – минимизация операцион ной травмы и уменьшение времени операции,сниже ние кровопотери, оптимизация периоперационного режима питания, ранняя мобилизация, меры реаби литации способствуют уменьшению выраженности послеоперационной инсулинорезистентности.
Оценка инсулинорезистентности проводится ме тодом гиперинсулинемического-нормогликемического зажима (метод разработан R.A. DeFronzo): инсулин вводится с постоянной скоростью до достижения уровня, превышающего показатель натощак [37]. То лерантный тест, индекс оценки модели гомеостаза и индекс количественной проверки чувствительности к инсулину не продемонстрировали значимого влияния на оценку выраженности инсулинорезистентности. Определение уровня гликированного гемоглобина и индекса массы тела обладают слабой предсказательной способностью. Эффективной считается адаптивная модель оценки инсулинорезистентности: основываясь на частом измерении гликемии производится посто янная инфузия глюкозы с переменной скоростью. Чем выше скорость инфузии, тем менее выражена инсули норезистентность [38, 39].
Практическая значимость инсулинорезистентно сти заключается в прогнозировании исходов. Возрас тание инсулинорезистентности на 20% удваивает риски развития осложнения и послеоперационной ле тальности [39]. В связи с установлением патофизиоло гии процесса, в современной научной литературе стал использоваться термин «диабет травмы», и был сфор мулирован важный вывод: периоперационный контроль гликемии важнее факта предоперационной установки диагноза сахарного диабета [40].
Обмен белка. Белковый гомеостаз достигается балансом катаболизма и анаболизма. Хирургический стресс приводит к активации симпатической и де прессии парасимпатической нервной системы,что реализуется преобладанием катаболизма. Белки ске летной мускулатуры подвергаются протеолизу, обра зовавшиеся аминокислоты трансформируются печенью в эндогенную глюкозу [41]. На фоне дисбаланса между катаболизмом и анаболизмом происходит по теря структурного и функционального белков орга низма [42].
При выполнении плановой операции на органах брюшной полости пациенты теряют до 80 граммов азота, что эквивалентно почти двум с половиной килограммам скелетной мускулатуры. Септические и ожоговые пациенты ежедневно теряют до килограмма скелетных мышц. Инсулинорезистентность удваивает суточную потерю белка [43, 44]. Инсулинорезистент-ность на фоне парентерального питания усугубляет белковый дисбаланс, что приводит к быстрой атрофии мышц [45]. Атрофические изменения скелетной мускулатуры сохраняются в течение пяти-восьми лет после перенесенной операции [46]. Пациенты со сниженным весом, ведущие гиподинамичный образ жизни и возрастные пациенты с саркопенией являются группой риска по нарушению белкового гомеостаза [47]. Таким группам пациентов целесообразно проведение преабилитационных мероприятий, направленных на увеличение массы скелетной мускулатуры. Также пациентам с саркопенией целесообразно применение парентерального питания коротким курсом в раннем послеоперационном периоде [48].
Альбумин является «отрицательным» белком острой фазы. Под воздействием хирургического стресса снижается синтез альбумина, постепенно восстанавливающийся в послеоперационном периоде и возвращающийся к нормальным значениям примерно через 3-4 недели [49]. Роль альбумина в послеоперационном статусе у пациентов требует дальнейшего изучения.
Послеоперационная иммобилизация оказывает негативное влияние на состояние пациентов, усугубляя атрофию мышц уже через сутки неподвижности. Данный механизм более выражен у возрастных пациентов [50]. Пациенты с недоеданием и онкологические пациенты сравнительно медленнее восстанавливаются после хирургической травмы, страдают от повышенных рисков развития осложнений и послеоперационной летальности [51].
Возможно прогнозировать послеоперационный белковый дисбаланс, для этого требуется оценка исходного уровня катаболизма. Однако в настоящее время не существует достоверных и доступных методов оценки: антропометрические способы ограничены при асцитах и отеках; оценка белковых фракций плазмы крови становится недостоверной при хрони- ческих заболеваниях, инфекциях и ряде других патологических состояний; экскреция азота с мочой (один грамм азота – это 6,25 граммов белка, оценивается через мочевину) не позволяет установить именно катаболизм, так как может быть следствием нарушения анаболизма [52, 53].
Оптимальным считается метод трассировки меченных изотопами аминокислот, позволяющий динамически регистрировать обмен глюкозы и аминокислот в организме. Недостатком является низкая доступность метода [54].
Установлена прямая связь качества периопера-ционного питания на подавление катаболизма и стимулирования анаболизма белков. Таким образом, у пациентов группы риска требуется особенный контроль диеты [55].
Послеоперационная потеря белка приводит к замедлению ранозаживления, снижению иммунитета, развитию астении. Установлена прямая связь потери скелетной мускулатуры со сроками послеоперационной нетрудоспособности и рисками послеоперационной летальности [56].
ОСНОВНЫЕ ПРИНЦИПЫ КУРАЦИИ БОЛЬНЫХ ПО ПРОГРАММЕУСКОРЕННОГО ВЫЗДОРОВЛЕНИЯ
-
1. Коррекция метаболических нарушений является одним из ключевых направлений в периоперационной курации пациентов. Контроль инсулино-резистентности уменьшает гликозилирование тканей, корригирует гипергликемию, активирует анаболизм. Употребление углеводно-белковых смесей непосредственно перед операцией уменьшает риски развития послеоперационной инсулинорезистентности [57]. Раннее послеоперационное питание стимулирует выработку эндогенного инсулина, ингибирует протеолиз, облегчает синтез белка [58]. Достижение послеоперационного анаболизма ускоряет восстановление
-
2. Уменьшить выраженность инсулинорезистент ности и катаболизма возможно минимизацией опера ционной травмы. Это достигается уменьшением общих суммарных размеров хирургических доступов, отказом от разрезания мышц в пользу их разведения. Уменьшение количества пересеченных дерматомов за счет правильной ориентации доступа позволяет уменьшить боль в интра-и послеоперационном пе риодах. Также следует уменьшить манипуляции с тка нями и органами: снизить количество перехватов, отведений кишечника; уменьшить контакт с паренхи матозными органами; избегать повреждения бры жейки, сосудов и нервов там, где возможно; умень шить кровопотерю за счет сокращения мобилизации тканей и органов [61].
-
3. Применение эпидуральной анестезии в первые двое суток после операции уменьшает инсулинорези стентность, снижает выраженность гипергликемии и катаболизма [62].
-
4. Поддержание интраоперационной нормотер мии прямым (подогрев пациента) и опосредованным (подогрев газов и растворов) способами позволяет снизить катехоламиновый ответ на стресс,снизить выраженность утери скелетной мускулатуры [63].
-
5. Послеоперационные физические упражнения способствуют активации анаболизма, улучшают метаболизм глюкозы и уменьшают резистентность к инсулину. Поэтому адекватное послеоперацион ное обезболивание в сочетании с ранней мобилиза цией позволяют достичь скорейшего выздоровле ния [64].
и уменьшает риски развития осложнений.Соответ ственно основная задача в раннем послеоперацион ном периоде – адекватная гидратация, ранняя моби лизация и энтеральное питание [59]. Периоперацион ное введение инсулина целесообразно для поддержа ния гликемии на уровне от 6 до 8 ммоль на литр и для преодоления послеоперационной резистентности [60].
ЛИТЕ РАТУ РА/RE FERENCE S
Eur J Anaesthesiol Suppl 1995;10:31–4.
ЛИТЕ РАТУРА/REFE RENCE S removal. Br J Urol 1996;78:893-6.
Список литературы Ускоренное выздоровление. Актуальность, история, патофизиология
- Nanavati AJ, Prabhakar S. A comparative study of «fast-track» versus traditional perioperative care protocols in gastrointestinal surgeries. J Gastrointest Surg 2014;18:757-67. https://doi.org/10.1007/s11605-013-2403-2.
- Finn WF. Enhanced recovery from postischemic acute renal failure. Micropuncture studies in the rat. Circ Res 1980;46:440-8. https://doi.org/10.1161/01.res.46.3.440.
- Birch BR, Anson KM, Clifford E, Miller RA. Day-case surgery: enhanced recovery with flumazenil. J R Soc Med 1990;83:436-8. https://doi.org/10.1177/014107689008300709.
- Engelman RM, Rousou JA, Flack JE, Deaton DW, Humphrey CB, Ellison LH, et al. Fast-track recovery of the coronary bypass patient. Ann Thorac Surg 1994;58:1742-6. https://doi.org/10.1016/0003-4975(94)91674-8.
- Kehlet H. Synergism between analgesics. Ann Med 1995;27:259-62. https://doi.org/10.3109/07853899509031968.
- Kehlet H, Rosenberg J. Late post-operative hypoxaemia and organ dysfunction. Eur J Anaesthesiol Suppl 1995;10:31-4.
- M0iniche S, Bülow S, Hesselfeldt P, Hestbaek A, Kehlet H. Convalescence and hospital stay after colonic surgery with balanced analgesia, early oral feeding, and enforced mo -bilisation. Eur J Surg 1995;161:283-8.
- Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001;322:473-6. https://doi.org/10.1136/bmj.322.7284.473.
- Wareing M, Candler S. Piloting day-case surgery for prostate resection. Prof Nurse 1999;14:754-8.
- Brinkman MJ, Duffin J, Wilson SK, Delk JR. Fast track transurethral resection of the prostate: application of case map improves length of stay without compromising patient outcome. Nurs Case Manag 1997;2:115-21.
- Mueller EJ, Zeidman EJ, Desmond PM, Thompson IM, Optenberg SA, Wasson J. Reduction of length of stay and cost of transurethral resection of the prostate by early catheter removal. Br J Urol 1996;78:893-6. https://doi.Org/10.1046/j.1464-410x.1996.01614.x.
- Prasopsuk S, Tunruttanakul S. Safety of a first-day catheter removal after transurethral resection of the prostate (TURP): a propensity score-matched historical control study. Insight Urology 2021;42:40-5. https://doi.org/10.52786/isu.a.21.
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. https://doi.org/10.1001/jamasurg.2016.4952.
- Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385 Suppl 2:S11. https://doi.org/10.1016/ S0140-6736(15)60806-6.
- Chen Z-X, Liu A-HJ, Cen Y. Fast-track program vs traditional care in surgery for gastric cancer. World J Gastroenterol 2014;20:578-83. https://doi.org/10.3748/wjg.v20.i2.578.
- Relph S, Bell A, Sivashanmugarajan V, Munro K, Chigwidden K, Lloyd S, et al. Cost effectiveness of enhanced recovery after surgery programme for vaginal hysterectomy: a comparison of pre and post-implementation expenditures. Int J Health Plann Manage 2014;29:399-406. https://doi.org/10.1002/hpm.2182.
- Lilot M, Ehrenfeld JM, Lee C, Harrington B, Cannesson M, Rinehart J. Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: retrospective two-centre analysis. Br J Anaesth 2015;114:767-76. https://doi.org/10.1093/bja/aeu452.
- Polderman JA, Van Velzen L, Wasmoeth LG, Eshuis JH, Houweling PL, Hollmann MW, et al. Hyperglycemia and ambulatory surgery. Minerva Anestesiol 2015;81:951-9.
- Carli F, Galeone M, Gzodzic B, Hong X, Fried GM, Wykes L, et al. Effect of laparo-scopic colon resection on postoperative glucose utilization and protein sparing: an integrated analysis of glucose and protein metabolism during the fasted and fed States using stable isotopes. Arch Surg 2005;140:593-7. https://doi.org/10.1001/ archsurg.140.6.593.
- Eberhart LHJ, Graf J, Morin AM, Stief T, Kalder M, Lattermann R, et al. Randomised controlled trial of the effect of oral premedication with dexamethasone on hypergly-caemic response to abdominal hysterectomy. Eur J Anaesthesiol 2011;28:195-201. https://doi.org/10.1097/EJA.0b013e32834296b9.
- Hatzakorzian R, Bui H, Carvalho G, Shan WLP, Sidhu S, Schricker T. Fasting blood glucose levels in patients presenting for elective surgery. Nutrition 2011;27:298-301. https://doi.org/10.1016Zj.nut.2010.02.003.
- Schricker T, Lattermann R, Wykes L, Carli F. Effect of i.v. dextrose administration on glucose metabolism during surgery. JPEN J Parenter Enteral Nutr 2004;28:149-53. https://doi.org/10.1177/0148607104028003149.
- Karon BS, Donato LJ, Larsen CM, Siebenaler LK, Wells AE, Wood-Wentz CM, et al. Accuracy of capillary and arterial whole blood glucose measurements using a glucose meter in patients under general anesthesia in the operating room. Anesthesio-logy 2017;127:466-74. https://doi.org/10.1097/ALN.0000000000001708.
- Rice MJ, Pitkin AD, Coursin DB. Review article: glucose measurement in the operating room: more complicated than it seems. Anesth Analg 2010;110:1056-65. https://doi.org/10.1213/ANE.0b013e3181cc07de.
- Ross-Huot M-C, Laferriere A, Gi CM, Khorashadi M, Schricker T, Coderre TJ. Effects of glycemic regulation on chronic postischemia pain. Anesthesio-logy 2011;115:614-25. https://doi.org/10.1097/ALN.0b013e31822a63c9.
- Shohat N, Muhsen K, Gilat R, Rondon AJ, Chen AF, Parvizi J. Inadequate glycemic control is associated with increased surgical site infection in total joint arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2018;33:2312-21.e3. https://doi.org/10.1016/j.arth.2018.02.020.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyper-glycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978-82. https://doi.org/10.1210/ jcem.87.3.8341.
- Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg 2013;257:8-14. https://doi.org/10.1097/ SLA.0b013e31827b6bbc.
- Hermanides J, Qeva E, Preckel B, Bilotta F. Perioperative hyperglycemia and neurocog-nitive outcome after surgery: a systematic review. Minerva Anestesiol 2018;84:1178-88. https://doi.org/10.23736/S0375-9393.18.12400-X.
- Abdelmalak BB, Knittel J, Abdelmalak JB, Dalton JE, Christiansen E, Foss J, et al. Preoperative blood glucose concentrations and postoperative outcomes after elective non-cardiac surgery: an observational study. Br J Anaesth 2014;112:79-88. https://doi.org/10.1093/bja/aet297.
- Ertuglu LA, Elijovich F, Laffer CL, Kirabo A. Salt-Sensitivity of Blood Pressure and Insulin Resistance. Front Physiol 2021;12:793924. https://doi.org/10.3389/ fphys.2021.793924.
- Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism 1978;27:1893-902. https://doi.org/10.1016/ s0026-0495(78)80007-9.
- Кобылянский ВИ. Роль контринсулярных гормонов в регуляции гомеостаза глюкозы и патогенезесахарного диабета 2-го типа при ХОБЛ. Проблемы Эндокринологии 2021;67:93-101. [Kobylyansky V.I. The role of counterinsular hormones in the regulation of glucose homeostasis and the pathogenesis of type 2 diabetes mellitus in COPD. Problemi Endocrinologi = Problems of Endokrinoljgy 2021;67(2):93-101. (In Russian)]. https://doi.org/10.14341/probl12566.
- Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care 1999;2:69-78. https://doi.org/10.1097/00075197-199901000-00012.
- Bagry HS, Raghavendran S, Carli F. Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology 2008;108:506-23. https://doi.org/10.1097/ ALN.0b013e3181649314.
- Wang ZG, Wang Q, Wang WJ, Qin HL. Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. Br J Surg 2010;97:317-27. https://doi.org/10.1002/bjs.6963.
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214-223. https://doi.org/10.1152/ajpendo.1979.237.3.E214.
- Nakadate Y, Sato H, Sato T, Codere-Maruyama T, Matsukawa T, Schricker T. Body mass index predicts insulin sensitivity during cardiac surgery: a prospective observational study. Can J Anaesth 2018;65:551-9. https://doi.org/10.1007/ s12630-018-1081-7.
- Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 2010;95:4338-44. https://doi.org/10.1210/ jc.2010-0135.
- Vanhorebeek I, Van den Berghe G. Diabetes of injury: novel insights. Endocrinol Metab Clin North Am 2006;35:859-72, x. https://doi.org/10.1016/j.ecl.2006.09.002.
- Giannoudis PV, Dinopoulos H, Chalidis B, Hall GM. Surgical stress response. Injury 2006;37 Suppl 5:S3-9. https://doi.org/10.1016/S0020-1383(07)70005-0.
- Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591-600. https://doi.org/10.1001/jama.2013.278481.
- Kinney JM, Elwyn DH. Protein metabolism and injury. Annu Rev Nutr 1983;3:433-66. https://doi.org/10.1146/annurev.nu.03.070183.002245.
- Schricker T, Gougeon R, Eberhart L, Wykes L, Mazza L, Carvalho G, et al. Type 2 diabetes mellitus and the catabolic response to surgery. Anesthesiology 2005;102:320-6. https://doi.org/10.1097/00000542-200502000-00013.
- Donatelli F, Corbella D, Di Nicola M, Carli F, Lorini L, Fumagalli R, et al. Preoper-ative Insulin Resistance and the Impact of Feeding on Postoperative Protein Balance: A Stable Isotope Study. The Journal of Clinical Endocrinology & Metabolism 2011;96:E1789-97. https://doi.org/10.1210/jc.2011-0549.
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787-94. https://doi.org/10.1001/jama.2010.1553.
- Morais JA, Chevalier S, Gougeon R. Protein turnover and requirements in the healthy and frail elderly. JNutr Health Aging2006;10:272-83.
- Bozzetti F, Gavazzi C, Miceli R, Rossi N, Mariani L, Cozzaglio L, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr 2000;24:7-14. https://doi.org/10.1177/014860710002400107.
- Hülshoff A, Schricker T, Elgendy H, Hatzakorzian R, Lattermann R. Albumin synthesis in surgical patients. Nutrition 2013;29:703-7. https://doi.org/10.1016/ j.nut.2012.10.014.
- Brower RG. Consequences of bed rest. Critical Care Medicine 2009;37:S422-8. https://doi.org/10.1097/CCM.0b013e3181b6e30a.
- Jagoe RT, Goodship TH, Gibson GJ. The influence of nutritional status on complications after operations for lung cancer. Ann Thorac Surg 2001;71:936-43. https://doi.org/10.1016/s0003-4975(00)02006-3.
- Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. Am J Clin Nutr 2012;96:591-600. https://doi.org/10.3945/ ajcn.111.032078.
- Prelack K, Dwyer J, Yu YM, Sheridan RL, Tompkins RG. Urinary urea nitrogen is imprecise as a predictor of protein balance in burned children. J Am Diet Assoc 1997;97:489-95. https://doi.org/10.1016/S0002-8223(97)00127-2.
- Berg A, Rooyackers O, Bellander B-M, Wernerman J. Whole body protein kinetics during hypocaloric and normocaloric feeding in critically ill patients. Crit Care 2013;17:R158. https://doi.org/10.1186/cc12837.
- Schricker T, Wykes L, Meterissian S, Hatzakorzian R, Eberhart L, Carvalho G, et al. The anabolic effect of perioperative nutrition depends on the patient's catabolic state before surgery. Ann Surg 2013;257:155-9. https://doi.org/10.1097/SLA.0b013e31825ffc1f.
- Christensen T, Bendix T, Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. Br J Surg 1982;69:417-9. https://doi.org/10.1002/bjs.1800690721.
- Ljungqvist O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract Res Clin Anaesthesiol 2009;23:401-9. https://doi.org/10.1016/j.bpa.2009.08.004.
- Hill GL, Douglas RG, Schroeder D. Metabolic basis for the management of patients undergoing major surgery. World J Surg 1993;17:146-53. https://doi.org/10.1007/BF01658920.
- Martindale RG, McClave SA, Taylor B, Lawson CM. Perioperative nutrition: what is the current landscape? JPEN J Parenter Enteral Nutr 2013;37:5S-20S. https://doi.org/10.1177/0148607113496821.
- Blixt C, Ahlstedt C, Ljungqvist O, Isaksson B, Kalman S, Rooyackers O. The effect of perioperative glucose control on postoperative insulin resistance. Clin Nutr 2012;31:676-81. https://doi.org/10.1016/jxlnu.2012.02.011.
- Kim TK, Yoon JR. Comparison of the neuroendocrine and inflammatory responses after laparoscopic and abdominal hysterectomy. Korean J Anesthesiol 2010;59:265-9. https://doi.org/10.4097/kjae.2010.59.4.265.
- Lugli AK, Donatelli F, Schricker T, Wykes L, Carli F. Epidural analgesia enhances the postoperative anabolic effect of amino acids in diabetes mellitus type 2 patients undergoing colon surgery. Anesthesiology 2008;108:1093-9. https://doi.org/10.1097/ ALN.0b013e3181730239.
- Carli F, Webster J, Nandi P, MacDonald IA, Pearson J, Mehta R. Thermogenesis after surgery: effect of perioperative heat conservation and epidural anesthesia. Am J Physiol 1992;263:E441-447. https://doi.org/10.1152/ajpendo.1992.263.3.E441.
- Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci 2008;63:1076-81. https://doi.org/10.1093/gerona/63.10.1076.


