Development of a methodology for obtaining herbal pharmaceutical substances for the production of phytoproducts
Автор: Ibrayeva M.B., Ryssalova B.B., Sabyrova A.B., Zhumashova G.T., Sakipova Z.B., Ridvanov Ch.I., Orynbekova S.O., Sermukhamedova O.V., Jakiyanov A.M., Turuspayeva Zh.Zh.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Технология пищевой и перерабатывающей промышленности
Статья в выпуске: 4 (150), 2025 года.
Бесплатный доступ
Modern trends in the pharmaceutical industry are characterized by a steady increase in interest in herbal remedies, driven by increased attention to the safety and efficacy of drugs, as well as the global desire to use environmentally friendly and renewable raw materials. Under these conditions, the development of scientifically based technological approaches aimed at ensuring the stability of the composition and reproducibility of the quality of herbal products is of particular importance.This paper presents a methodology for obtaining medicinal herbal raw materials of pharmacopoeial quality, based on the application of the Quality by Design concept and the Design of Experiments tool. The studies were carried out using the above-ground parts of the plants Seseli sessiliflorum Schrenk, Dracocephalum bipinnatum Rupr., Dracocephalum integrifolium Bunge, Dracocephalum ruyschiana L. and Ziziphora bungeana Juz., which are characterized by a high content of biologically active components, including essential oils, flavonoids and phenolic compounds with pronounced biological activity. The study assessed the influence of harvesting, drying, grinding, and storage parameters on the preservation of heat-labile components and the stability of the chemical profile of the studied raw materials. The developed methodology for obtaining plant raw materials holds great promise for the subsequent development of drugs, dietary supplements, and cosmetics with predictable quality and effectiveness, as well as for improving the standardization and quality control system for plant materials in accordance with international requirements.
Medicinal plant raw material, infrared radiation, essential oil, design of experiments, parameters of technological process, quality of the pharmaceutical product
Короткий адрес: https://sciup.org/140313213
IDR: 140313213 | УДК: 34.45.00; 65.01; 76.31 | DOI: 10.48184/2304-568X-2025-4-15-25
Текст научной статьи Development of a methodology for obtaining herbal pharmaceutical substances for the production of phytoproducts
Plant raw materials are complex biochemical systems containing a wide range of secondary metabolites – phenolic compounds, flavonoids, terpenes, coumarins, alkaloids, and other natural substances. These components provide a variety of biological effects, including antioxidant, antiinflammatory, antimicrobial, and cardioprotective actions, which determine the therapeutic potential of herbal preparations. The chemical composition of plant raw materials depends on the plant species, vegetation phase, climatic and environmental conditions, as well as the methods of collection, drying, and storage. Variations in these parameters affect the content and stability of biologically active substances, which affects the quality and reproducibility of pharmacological activity. Regulating the production and processing of plant raw materials is considered a key step in the development of herbal medicines, dietary supplements, and cosmetics based on them .
Representatives of the Apiaceae and Lamiaceae families are distinguished by their high level of chemical diversity and wide use in traditional and modern herbal medicine. Plants of the Apiaceae family are characterized by the accumulation of phenylpropanoids, coumarins, polyacetylenes and various terpene compounds, while Lamiaceae plants contain predominantly phenolic acids, flavonoids and aromatic monoterpenes, including linalool, thymol and carvacrol. The similarity in the structure of the main metabolic pathways and the recurrence of key groups of biologically active compounds create the preconditions for unifying approaches to studying these plants, optimizing the extraction processes, and standardizing biologically active components. This, in turn, forms the basis for the industrial production of herbal remedies, dietary supplements, and cosmetics of plant origin .
The relevance of herbal medicine and the production of herbal products is confirmed by the significant volume of the global market for herbal medicines, dietary supplements, and cosmetics. According to analytical estimates, the herbal medicine market was approximately $70.6 billion in 2023, with projected growth to $328.7 billion by 2030 (a Compound Annual Growth Rate of approximately 21% from 2024 to 2030). The dietary supplement market is estimated at approximately $192.7 billion in 2024 and is also demonstrating a steady expansion. The large-scale production and trade of herbal products necessitates strict quality assurance and reproducibility of raw materials: fluctuations in the content of biologically active substances between batches can reduce the therapeutic and functional effectiveness of finished products, which requires standardization of approaches to the collection and processing of herbal raw materials .
To ensure the consistent quality of herbal raw materials and the reproducibility of their characteristics, a systematic approach is necessary, including standardization of procurement and processing conditions, as well as the use of statistically validated methods for process optimization. This methodological framework ensures traceability and validity of production parameters, which is a key requirement for the standardization of herbal remedies.
In recent years, the principles of the Quality by Design (QbD) concept have been actively applied in pharmaceutical practice, including Design of Experiments (DoE) methods aimed at quality management by analyzing the relationships between process parameters and the properties of the final product. The use of QbD approaches facilitates the creation of predictive models, optimization of experimental studies, and ensuring the consistent quality of herbal products .
The aim of this work is to develop a methodology for obtaining medicinal herbal raw materials, including statistically validated optimization of processing parameters, with an emphasis on ensuring the preservation of key classes of bioactive substances and the applicability of the results for the further development of herbal medicinal products, dietary supplements, and cosmetics.
Materials and methods
The study subjects were the aboveground parts of plants – leaves, flowers (inflorescences), and stem tips – of the species Seseli sessiliflorum Schrenk , Dracocephalum bipinnatum Rupr ., Dracocephalum integrifolium Bunge, Dracocephalum ruyschiana L., and Ziziphora bungeana Juz.
Seseli sessiliflorum Schrenk – or Ziziphora bungeana – is a perennial herbaceous plant up to 70 cm tall, growing on dry, rocky and gravelly slopes, in foothill areas, and on the slopes of desert uplands. In folk medicine, the aboveground parts of S. sessiliflorum are used as an anti-inflammatory, antispasmodic, and antimicrobial agent.
Dracocephalum bipinnatum Rupr. is a perennial plant up to 50 cm tall, growing in Central Asia on rocky slopes and scree up to the upper mountain belt. In folk medicine, the above-ground part of D. bipinnatum is used as a sedative and hypotensive agent .
Dracocephalum integrifolium Bunge. is a perennial plant up to 60 cm tall, growing in Central Asia, Mongolia, and China on rocky, gravelly, and grassy slopes, in forests and shrubs, primarily in the lower mountain belt. In folk medicine, the aboveground part of D. integrifolium is used as a sedative, hypotensive, and recommended as an antiseptic.
Dracocephalum ruyschiana L. – Ruysch's dragonhead – is a perennial plant up to 60 cm tall, growing on rocky and meadow slopes, along the floodplains of mountain rivers, and in sparse forests. In folk medicine, the above-ground part of
D. ruyschiana is used as an astringent, antispasmodic, tonic, potency-enhancing, and wound-healing agent .
Ziziphora bungeana Juz. is a perennial herbaceous plant up to 40 cm high, growing on rocky and gravelly slopes, in steppe and foothill areas. In folk medicine, the above-ground part of Z. bungeana is used as an antiseptic, wound-healing, antiinflammatory and analgesic agent .
The raw materials were collected in the Almaty region, on the foothills of the Dzungarian Alatau, during the period of mass flowering of the plants. The species identification of the samples was confirmed by specialists from the Institute of Botany and Phytointroduction of the Republic of Kazakhstan.
The development of infrared (IR) drying technology for plant essential oil raw materials was carried out within the framework of the QbD concept. During the drying conditions optimization stage, the DoE statistical tool implemented in Minitab Statistical Software 21 was used. The following independent variables (factors, X) were selected: temperature: 35– 75°C; time: 60–360 min. The dependent variables were"essential oil yield" (% of dry weight, Y 1 ) and "flavonoid content" (mg/g, Y 2 ), characterizing the efficiency of the drying process and the degree of preservation of biologically active components.
Drying was carried out in a RAWMiD IR-1000 infrared drying chamber (RAWMiD, Republic of Kazakhstan/China), equipped with infrared ceramic emitters with a wavelength of 2–10 μm and a built-in digital temperature control system. The system ensured uniform heat flow distribution across the plant material layer, reducing the risk of localized overheating and preserving volatile components. The temperature in the drying chamber was maintained automatically with an accuracy of ±1°C; the thickness of the raw material layer on the pallet was maintained within 2–3 cm.
Results and discussion
The objects of the study were the aboveground parts of plants – leaves, flowers (inflorescences), stem tips, and vegetative-generative shoots of the species Seseli sessiliflorum Schrenk, Dracocephalum bipinnatum Rupr., Dracocephalum integrifolium Bunge, Dracocephalum ruyschiana L., and Ziziphora bungeana Juz. Collection was carried out at the beginning or full flowering stage, which corresponds to the period of maximum accumulation of biologically active substances and the accepted practice of harvesting flowering raw materials.
Collection was carried out in the morning hours (8–11 am) in dry, clear weather, avoiding high humidity and precipitation to prevent microbiological contamination. Plants were cut with pruning shears at a height of 20–25 cm from the ground, removing damaged and contaminated parts. For each species, three independent replicates of 400–500 g of fresh material were collected from different sites representative of the species' range. The description included the sample code, species, date and time of collection, coordinates, altitude, habitat type, and plant community characteristics.
Each package and documentation was assigned a unique sample code, indicating the species, code, date and time of collection, GPS coordinates, altitude, developmental stage, weight, and the names of the individuals who collected the samples. The collected samples were placed in paper bags, labeled, and delivered to the laboratory on the day of collection, avoiding exposure to sunlight and excessive heat.
After harvesting the plants, a commodity analysis of the fresh plant material was conducted in accordance with the requirements of regulatory and technical documentation governing the quality and authenticity of medicinal plant materials .
The commodity analysis included an assessment of organoleptic and diagnostic characteristics (appearance, color, odor, leaf and inflorescence morphology), as well as checking the purity of the material and the absence of impurities, damage, and signs of spoilage.
Based on the combined analyses, it was established that the collected plant material meets quality requirements and is suitable for subsequent study of drying parameters.
The development of a drying technology for medicinal plant materials is aimed at effectively removing moisture while preserving biologically active and heat-labile compounds. IR drying was chosen as the method, ensuring rapid and uniform heating of the material and reducing the duration of the process.
Optimization of the drying process parameters was carried out using the QbD concept, which utilized the statistical design of experiments (DoE) tool. The use of DoE allowed us to evaluate the impact of drying temperature and time on key quality indicators and justify the selection of optimal parameter values .
A central composite design with 13 experimental points was implemented over a temperature range of 35–75°C and a time of 60– 360 min. Data processing in Minitab Statistical Software 21 allowed us to identify patterns in the influence of these factors and determine optimal process parameters. IR radiation parameters were maintained constant: wavelength 1–5 μm, radiation intensity 0.5–0.8 W/cm², and distance from the source to the sample surface 10–20 cm.
Table 1. Results of the DoE model study for the quality indicators “Essential oil content” and “Flavonoid content” in plants
|
R u nOr de r |
X |
Y 1 |
Y 2 |
|||||||||
|
Temp ., °C |
Time, h |
Essential oil content, % |
Flavonoid content, mg/g |
|||||||||
|
S. sessil iflorum |
D. ruys chiana |
D. inte grifoliu m |
D. bipi nnatu m |
Z. bun geana |
S. sessi lifloru m |
D. ruys chiana |
D. integ rifolium |
D. bipi nnatu m |
Z. bu ngea na |
|||
|
1 |
35.0 |
6.0 |
0.150 |
0.190 |
0.165 |
0.200 |
0.278 |
7.8 |
11.5 |
9.7 |
9.5 |
26.5 |
|
2 |
26.7 |
3.5 |
0.042 |
0.048 |
0.045 |
0.055 |
0.062 |
5.2 |
7.5 |
6.4 |
6.3 |
18.0 |
|
3 |
75.0 |
6.0 |
0.006 |
0.005 |
0.004 |
0.005 |
0.006 |
3.8 |
5.9 |
5.0 |
4.9 |
14.0 |
|
4 |
55.0 |
3.5 |
0.352 |
0.430 |
0.365 |
0.438 |
0.545 |
10.6 |
15.3 |
12.8 |
12.7 |
34.8 |
|
5 |
55.0 |
3.5 |
0.348 |
0.423 |
0.359 |
0.426 |
0.538 |
10.7 |
15.4 |
12.9 |
12.8 |
34.9 |
|
6 |
75.0 |
1.0 |
0.091 |
0.112 |
0.098 |
0.117 |
0.142 |
4.8 |
7.0 |
6.0 |
5.9 |
15.5 |
|
7 |
55.0 |
3.5 |
0.351 |
0.427 |
0.362 |
0.432 |
0.541 |
10.8 |
15.5 |
13.0 |
12.9 |
35.0 |
|
8 |
35.0 |
1.0 |
0.031 |
0.036 |
0.032 |
0.037 |
0.041 |
5.9 |
8.2 |
7.0 |
6.9 |
19.5 |
|
9 |
55.0 |
0.5 |
0.071 |
0.089 |
0.083 |
0.093 |
0.108 |
7.4 |
10.5 |
8.9 |
8.8 |
25.0 |
|
10 |
55.0 |
3.5 |
0.353 |
0.429 |
0.361 |
0.435 |
0.543 |
10.9 |
15.6 |
13.1 |
13.0 |
35.1 |
|
11 |
55.0 |
7.04 |
0.247 |
0.305 |
0.258 |
0.312 |
0.386 |
8.8 |
12.6 |
10.7 |
10.6 |
29.0 |
|
12 |
55.0 |
3.5 |
0.349 |
0.424 |
0.357 |
0.428 |
0.539 |
10.5 |
15.2 |
12.7 |
12.6 |
34.7 |
|
13 |
83.3 |
3.5 |
0.004 |
0.005 |
0.003 |
0.006 |
0.004 |
3.5 |
5.2 |
4.6 |
4.4 |
12.0 |
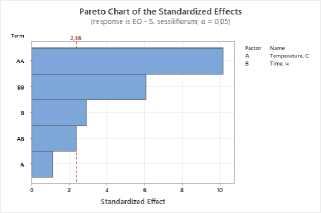
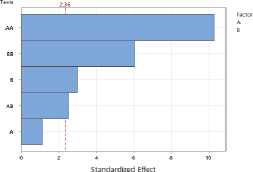
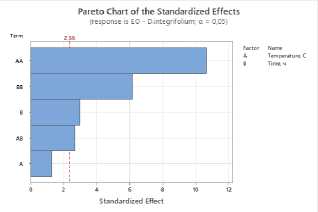
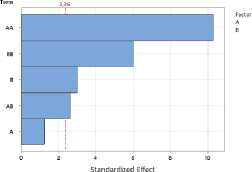
To assess the impact of process parameters on the yield of essential oil and flavonoids in raw materials, a Pareto plot of standardized effects was constructed. The plot shows the standardized effect values of the drying temperature (A) and drying time (B) factors, as well as their quadratic (AA, BB) and interaction (AB) terms.
a)
Pareto Chart of the Standardized Effects (response is EO - D. ruyschiana; a = 0.05)
b)
c)
Pareto Chart of the Standardized Effects (response is EO - D. bipinnatum; a = 0,05)
d)
Pareto Chart of the Standardized Effects (response is EO - Z. bungeana; a = 0,05)
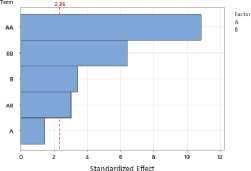
e)
Figure 1. Pareto plots of standardized effects of drying temperature and time factors on essential oil content: a – S. sessiliflorum ; b – D. ruyschiana ; c – D. integrifolium ; d – D. bipinnatum ; e – Z. bungeana . EO – essential oil.
Data analysis revealed that the quadratic effect of temperature (AA) has the greatest influence on essential oil yield, indicating a nonlinear relationship: at extremely low or high temperatures, yield decreases, while the maximum is achieved at intermediate values (Figure 1). The quadratic effect of time (BB) was also significant, indicating the existence of an optimal drying time range. The linear effects of temperature (A), time (B), and their interaction (AB) were less pronounced and statistically insignificant. Thus, the determining factors in essential oil yield are temperature-time parameters in their nonlinear relationship, with optimal conditions corresponding to the average values of the factors.
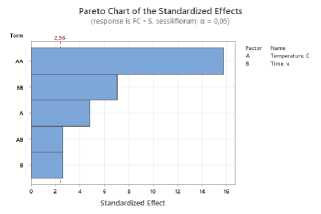
a)
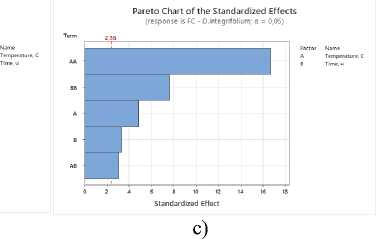
Pareto Chart of the Standardized Effects (response is FC ■ Z. bunqeana; a = 0,05)
Pareto Chart of the Standardized Effects {response is FC - D. ruyschiana; a = 0,05)
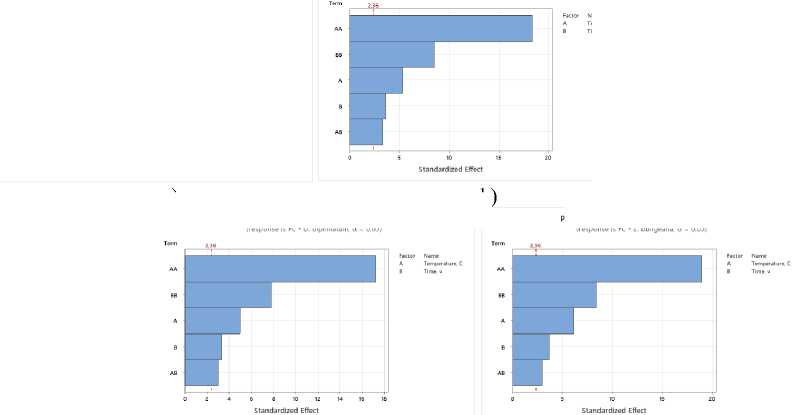
d)
e)
Figure 2. Pareto plots of standardized effects of drying temperature and time factors on flavonoid content: a – S. sessiliflorum ; b – D. ruyschiana ; c – D. integrifolium ; d – D. bipinnatum ; e – Z. bungeana FC – flavonoid content.
Pareto Chart of the Standardized Effects (response is FC - D. bipinnatum; a = 0,35)
b)
Based on the Pareto diagram analysis for all samples studied, it was found that drying temperature and time have a significant impact on the total flavonoid content (Figure 2). A significant influence is observed from the temperature regime in its quadratic form, indicating a nonlinear relationship: as the temperature increases, the yield of flavonoids increases to a certain limit, after which it decreases due to thermal degradation of the compounds. The duration of the drying process also influences the result, determining the completeness of drying and the preservation of heat-sensitive components.
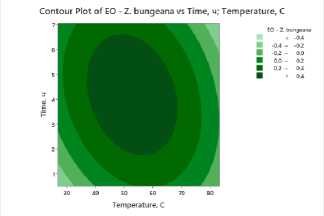
To clearly visualize the impact of process factors – temperature and drying time – on essential oil and flavonoid content, contour plots were constructed for all studied plants . The plots show the distribution of essential oil yield values as a function of temperature (X-axis) and drying time (Y-axis). Isolines represent response levels, and color gradation indicates the intensity of change in essential oil content: darker areas correspond to maximum values, while lighter areas indicate low yield.
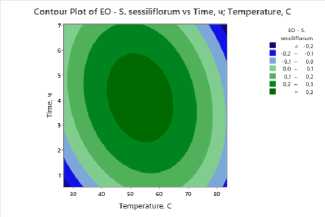
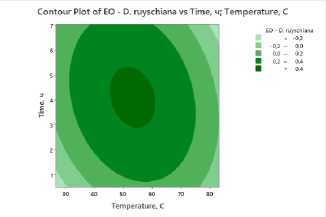
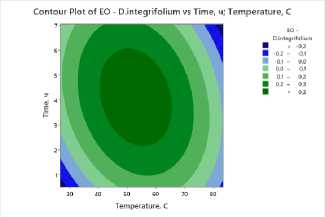
a) b) c)
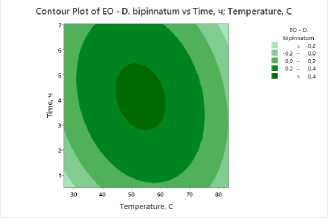
d)
e)
Figure 3. Contour and surface response plots demonstrating the effect of drying temperature and time on essential oil content: a – S. sessiliflorum ; b – D. ruyschiana ; c – D. integrifolium ; d – D. bipinnatum ; e – Z. bungeana .
EO – essential oil.
A common pattern was observed in all cases: high essential oil yields were observed at moderate temperatures (45–55°C) and drying times of 3.5–5 hours (Figure 3). At low temperatures, dehydration is incomplete, leading to moisture retention and incomplete release of biologically active components. However, at temperatures above 70°C and drying times exceeding 6 hours, degradation of heat-labile compounds and loss of volatile substances occur. Contour plots for all samples confirm the existence of an optimal parameter range ensuring maximum essential oil yield. The effectiveness of IR drying is determined by the balance between temperature and time, which ensures the preservation of biologically active substances and a high concentration of essential oil.
d)
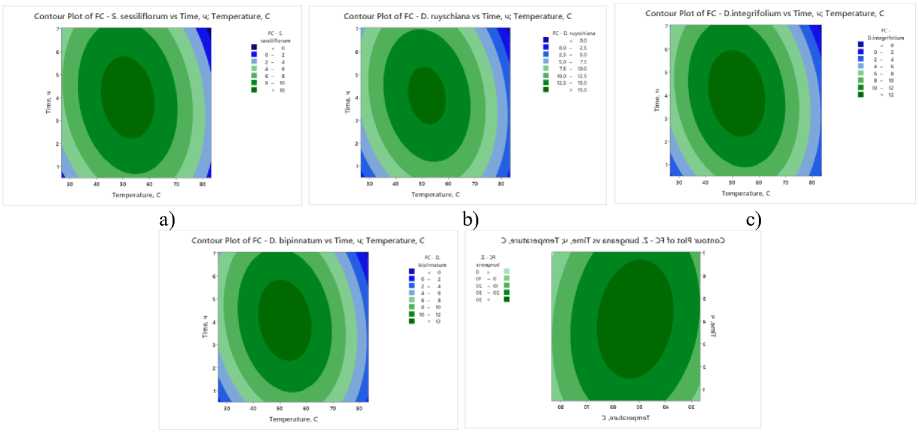
e)
Figure 4. Contour and surface response plots demonstrating the effect of drying temperature and time on flavonoid content yield: a – S. sessiliflorum ; b – D. ruyschiana ; c – D. integrifolium ; d – D. bipinnatum ; e – Z. bungeana .
FC – flavonoid content.
Contour graphs of the studied samples show similar dynamics of changes in the content of total flavonoids depending on the temperature and drying time (Figure 4). Maximum values are achieved at moderate temperatures of 45–55°C and process durations of 3.5–5 hours, which ensures an optimal combination of moisture removal rate and preservation of heat-labile substances. At low temperatures and short drying times, the release of active compounds is limited, whereas exceeding 70°C and increasing the drying time beyond 6 hours leads to their partial degradation and a decrease in the total flavonoid content.
To determine the optimal parameters for IR drying of the essential oil plants under study, a response analysis was conducted. The graphs show the dependence of the yield of flavonoids and essential oil on the temperature and drying time, as well as the calculated values of the function for each plant species. The generalized desirability indices were D=0.9922 (for flavonoids) and D=0.9872 (for essential oils), which indicates a high degree of optimality of the selected conditions.
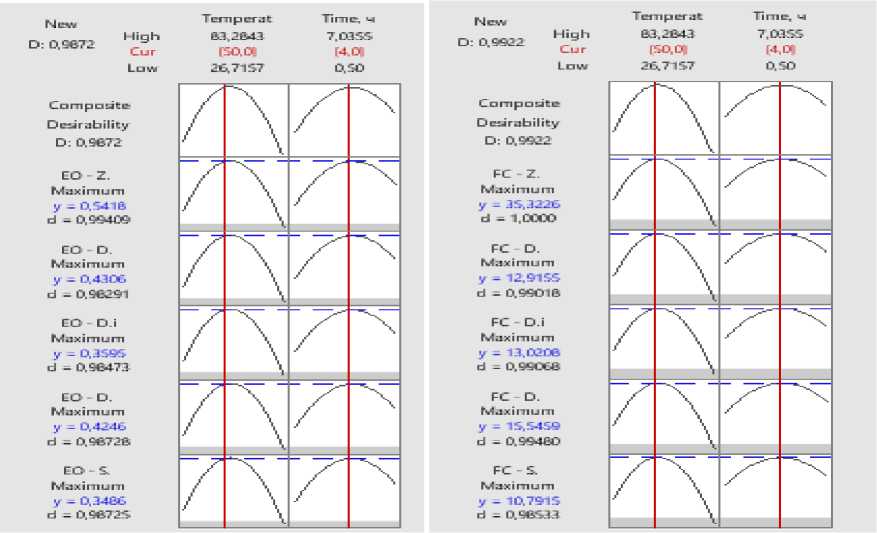
Figure 5. Graphs of optimization of temperature and drying time factors for the content of essential oil and flavonoids in plants: S. sessiliflorum ; D. bipinnatum ; D. integrifolium ; D. ruyschiana ; Z. bungeana . EO – essential oil, FC – flavonoid content.
An analysis of the graphs revealed that both temperature and drying time have a nonlinear effect on the studied parameters. With increasing temperature and drying time, the yield of active substances increases to a certain point, after which the values decrease due to the thermolability of the compounds (Figure 5).
Based on the constructed contour and optimization graphs, the optimal range of process parameters for all studied species lies in the temperature range of 45–55°C and drying times of 3.5–5.0 hours. The center of the optimum, according to the graph data, corresponds to a temperature of – 50°C and a drying time of –4.0 hours. Within these parameter ranges, the model indicates the maximum content of essential oil and flavonoids.
After drying, the plant materials were ground to a medium (1.0 cm) or fine (0.5 cm) fraction, or used whole. The dried raw materials were packaged in three-layer kraft paper bags (50×25 cm) of uniform weight, providing protection from moisture, light, and contamination. The packaging and filling conditions complied with the requirements for primary containers for medicinal plant materials. Each package was labeled with the manufacturer's name, medicinal product, batch number, packaging date, net weight, storage conditions, and expiration date.
Stability tests and shelf life determination were carried out in accordance with the Order of the Ministry of Health of the Republic of Kazakhstan dated October 28, 2020 No. KR DSM-165/2020. Three batches of each raw material were used for the study. Storage conditions: temperature not exceeding 25°C, relative humidity 60±5%. The testing program included an assessment of organoleptic properties, identification, moisture content, foreign matter, total
|
ash, ash insoluble in 10% hydrochloric acid, medicinal plant materials was developed, covering a microbiological purity, and quantitative content of range of activities – from collecting and processing biologically active substances. raw materials to obtaining standardized material As a result of the research and optimization of suitable for further pharmaceutical use. The drying parameters, a technology for collecting and methodology algorithm, presented in Figure 6, processing medicinal plant materials was developed, reflects the sequence of key stages: collection, encompassing stages from procurement to obtaining processing, drying, grinding, packaging, and storage, finished standardized material. The developed process ensuring the preservation of biologically active flow diagram is shown in Figure 4, reflecting the components and compliance with quality and sequence of stages: collection, primary processing, traceability requirements for each batch of raw drying, grinding, packaging, and storage. materials. As a result of the research and optimization of drying parameters, a methodology for obtaining |
||||
|
1. Determination of the research object |
||||
|
8 в е 'а £ 8 8 ОХ 3 |
Aboveground plant parts: leaves, flowers (inflorescences), stem tips, vegetative-generative shoots of the species S. sessiliflorum; D. ruyschiana; D. integrifolium; D. bipinnatum; Z. bungeana |
|||
|
2. Determination the collection phase |
||||
|
Collect during the early or full flowering phase. |
||||
|
3. Choosing the time and conditions |
||||
|
Morning hours (8–11 AM), dry, clear weather; avoid high humidity and precipitation. |
||||
|
4. Plant collection |
||||
|
Cut with pruning shears at a height of 20–25 cm; remove damaged and contaminated parts; form three replicas of 400–500 g each from different sites. |
||||
|
5. Documentation |
||||
|
Assign a unique code to the sample, indicate the species, date/time, GPS coordinates, developmental stage, weight, and names of the collectors. |
||||
|
6. Transport to the laboratory |
||||
|
Place raw materials in paper bags, avoiding exposure to sunlight and excessive heat |
||||
|
7. Commodity analysis |
||||
|
Assessment of organoleptic and diagnostic characteristics, check for purity, damage, and impurities |
||||
|
8. Drying |
||||
|
IR drying, temperature 45–55 °C, time 3.5–5 h, wavelength 1–5 μm, intensity 0.5–0.8 W/cm², distance 10–20 cm. |
||||
|
9. Grinding |
||||
|
Obtaining a medium (1 cm) or fine (0.5 cm) fraction or using the whole material. |
||||
|
10. Package |
||||
|
Three-layer kraft paper bags (50×25 cm), protection from moisture, light, and contamination; labeling indicating the name, batch, date, weight, storage conditions, and expiration date |
||||
|
11. Stability and storage testing |
||||
|
Three batches of each raw material, storage conditions: ≤25°C, 60±5% humidity; assessment of organoleptic properties, identification, moisture, foreign matter, microbiological purity, and biologically active substance content |
||||
Figure 6. Algorithm of the methodology for obtaining medicinal plant raw materials
Conclusion
As a result of the study, a methodology for obtaining pharmacopoeial-grade medicinal plant material was developed. This methodology included collecting the aerial parts of S. sessiliflorum, D. bipinnatum, D. integrifolium, D. ruyschiana, and Z. bungeana plants. To optimize the drying process parameters, a QbD approach was applied using the Design of Experiment (DoE) method in Minitab software. This approach allowed us to establish statistically significant relationships between temperature, process duration, and the preservation of biologically active compounds, determine optimal values for critical parameters, and ensure reproducible quality of the finished plant material. The developed methodology complies with the principles of good manufacturing practice and ensures the production of plant material suitable for the further development of medicinal products, dietary supplements, and cosmeceuticals. The obtained results provide the basis for the systematic implementation of QbD principles in the processes of procurement and processing of medicinal plant materials, as well as for further research aimed at increasing their pharmacological and technological potential.
Funding
This article was prepared within the framework of the intramural grant project of S.D. Asfendiyarov Kazakh National Medical University “Pharmaceutical development of oral care products based on plant substances for orthodontics.”


