Modifying cotton fabrics with copper nanoparticles to impart antibacterial properties
Автор: Baimakhanova M.B., Jurinskaya I.M., Taussarova B.R., Onggar T.
Журнал: Вестник Алматинского технологического университета @vestnik-atu
Рубрика: Технология текстиля и одежды, дизайн
Статья в выпуске: 3 (149), 2025 года.
Бесплатный доступ
This article is dedicated to the development of antimicrobial cellulose textile materials based on copper nanoparticles, obtained following the principles of «green chemistry». The main goal of the research was to create an environmentally friendly method for modifying cotton fabrics, ensuring a stable antibacterial effect. Ascorbic acid was used as a reducing agent, while gelatin and glucose served as stabilizers. This choice allowed for the formation of stable colloidal systems of copper nanoparticles and their uniform distribution within the fibrous structure. To enhance the fixation of the nanoparticles, tetraethoxysilane was additionally used to form a silicon oxide matrix. The paper describes the synthesis and modification methods for cotton fabrics and analyzes the properties of the selected stabilizers. Scanning electron microscopy confirmed the differences in nanoparticle morphology: using gelatin resulted in smaller and more uniform particles (30–74 nm), whereas glucose led to the formation of larger aggregates (56– 143 nm). These data are consistent with the results of microbiological tests: the treated samples showed a zone of inhibition of E. coli growth up to 0.4 mm. The practical value of the research lies in the development of a method that can be used to create functional cotton fabrics with antimicrobial and protective properties, which are in demand in the light and medical industries.
Cellulose textile materials, modification, green chemistry, copper nanoparticles, antibacterial properties
Короткий адрес: https://sciup.org/140312214
IDR: 140312214 | УДК: 64.29.23 | DOI: 10.48184/2304-568X-2025-3-269-278
Текст научной статьи Modifying cotton fabrics with copper nanoparticles to impart antibacterial properties
МРНТИ 64.29.23
Introduction.
In recent years, against the backdrop of the rapid development of nanotechnology, there has been growing interest in creating textile materials modified with metal nanoparticles. Metal nanoparticles possess unique properties that differ from the properties of the bulk metal from which they are derived [1]. Such nanocomposite textile systems combine the physicochemical characteristics of the fibrous structure with the functional properties of the nanoparticles, ensuring their durability and stability within the fabric matrix.
Particular attention is given to copper nanoparticles, which exhibit pronounced biological and antimicrobial activity [2]. As microorganisms develop resistance to traditional antibiotics, the development of alternative antibacterial solutions based on metal nanoparticles is becoming especially relevant. Nanoparticles of copper and copper oxide demonstrate a broad spectrum of action and can complement traditional antimicrobial agents [3, 4]. Modern research increasingly relies on the principles of «green chemistry», which involve the development of environmentally friendly methods for synthesizing functional nanomaterials [5]. The use of «green» approaches in organic chemistry laboratory practice helps reduce the use of hazardous reagents and lessen the environmental impact.
The implementation of such methods in the textile industry, particularly in the modification of cotton materials, is becoming especially relevant. Cotton was used as the base due to its widespread use and valuable performance properties such as hygroscopicity, softness, and biodegradability. However, it also provides a favorable environment for the growth of pathogenic microorganisms, including bacteria, fungi, and viruses [6]. Therefore, the creation of hygienic antimicrobial fabrics based on cotton is important for preventing the spread of infections.
The proposed method is based on the synthesis of copper nanoparticles using natural stabilizers (gelatin and glucose) and ascorbic acid as a reducing agent. The additional introduction of tetraethoxysilane ensures the formation of a silicon oxide matrix, which contributes to a stronger fixation of the nanoparticles in the fiber structure and increases the durability of the antimicrobial effect. The novelty of this work lies in combining the green synthesis of copper nanoparticles with the creation of a silicate matrix on the surface of cotton fabrics.
The aim of the study is to develop an environmentally safe method of modification of cotton fabrics with copper nanoparticles for the formation of stable antimicrobial properties, the objectives include the synthesis of copper nanoparticles using ascorbic acid as a reducing agent and natural stabilisers (gelatin and glucose), the study of the influence of the selected stabilisers on the morphology of particles, the modification of cotton fabrics with the formation of silicate matrix, the assessment of their antibacterial activity, as well as a comparative study of the antibacterial effect of the modified fabrics. The hypothesis of the study is that the use of natural stabilisers will allow to obtain stable copper nanoparticles distributed in the structure of fibres, which will provide long-term antibacterial effect of modified fabrics.
A brief literature review on the development of antibacterial textile materials modified with copperbased nanoparticles. Savelieva Y.I. et al. investigated antibacterial polyurethane composites containing silver and copper nanoparticles, noting their high antimicrobial efficacy and potential application in functional materials [7]. Sheikh A., Nambi R., and Sayeda M.A. (2022) demonstrated that green-synthesized copper nanoparticles from Cocculus hirsutus possess pronounced antioxidant, antibacterial, and antidiabetic properties, highlighting the biomedical significance of copper-based nanomaterials [8]. Emrini M.L. and Valiani V. (2021) provided a comprehensive review of copper-containing nanoagents, emphasizing the unique antimicrobial potential of copper and its oxides [9]. Their work traces a growing interest in copper nanostructures, which researchers often characterize as a kind of «copper stage» in the development of antimicrobial nanotechnology.
Recent studies have identified environmentally friendly methods for imparting antibacterial properties to cotton fabrics using copper-based nanoparticles. For example, Mehrabani B. et al. (2022) developed an ecofriendly in situ synthesis of copper nanoparticles on polyester fabrics using chitosan and ascorbic acid, demonstrating effective antimicrobial properties and suitability for use in textiles [10]. Pérez-Álvarez et al. (2022) reported a simple and inexpensive method for synthesizing copper nanoparticles directly from cotton fibers, avoiding the use of toxic reducing agents and yielding materials with promising antimicrobial properties [11].
Another study in ACS Applied Nano Materials (2022) described coatings of copper nanoparticles (both CU₂O and metallic Cu, ≈50–150 nm) on fabrics commonly used in masks and cleaning wipes. These coatings exhibit rapid and stable antibacterial activity, killing pathogens such as S. aureus, K. pneumoniae, and P. aeruginosa in as little as 45 seconds [12].
Textile materials have a wide range of applications in the home, industry, transport and as special protective equipment [13]. Also, it should be noted that the group of cellulose-containing textile materials is of the greatest interest, as these fabrics and fibres have unique properties [14]. On the other hand, natural fibres, polymers and bio-based fillers are fully degradable materials [15].
Evaluating these achievements, it becomes clear that creating durable antimicrobial cotton fabrics requires not only powerful bacterial inactivation but also stable immobilization of nanoparticles and the preservation of fabric integrity. Therefore, key research areas include developing reliable fixation methods, ensuring environmental safety, and assessing the long-term functional stability of modified textiles.
Materials and methods
This study focuses on the development and safety evaluation of textile materials with antimicrobial properties. For this purpose, objects were selected that represent both the base material and the modifying reagents needed to achieve the desired properties, in accordance with current scientific literature.
Objects of Study:
Cotton Fabric - Article 1030. The fabric size used in the experiment was 20×20 cm, with a surface density of 130 g/m2. The breaking load was 315 mm (warp) and 346 mm (weft). Cotton fabric was chosen as the main substrate because it is widely used in the light and medical industries due to its hygienic, mechanical, and sorptive properties. However, the natural nature of the fiber makes it vulnerable to microbial degradation.
Copper Sulfate — An inorganic compound with the formula CuSO4. The anhydrous form is a colorless, odorless, and highly hygroscopic substance that is readily soluble in water. Its molar mass is 159.60 g/mol and its density is 3.64 g/cm3. It possesses disinfectant and antiseptic properties
Gelatin — A natural polymer that acts as a biocompatible stabilizer in nanoparticle synthesis reactions.
Ascorbic Acid (С6Н8О6 ) A white, crystalline powder with a sour taste, easily soluble in water and alcohol.
Glucose (С6 Н12 О6) – an organic compound, a colorless, odorless crystalline substance that also has reducing properties .
The synthesis of copper nanoparticles was performed using a «green chemistry» method, incorporating naturally derived reducing agents and stabilizers. Two different formulations were used, distinguished by the type of stabilizer: gelatin and glucose. Ascorbic acid served as the reducing agent, and copper (II) sulfate pentahydrate (CuSO-r5H>O) was the source of copper ions.
Synthesis with Gelatin:
An aqueous solution of CuSO-r5H>O was mixed with a gelatin solution, which acted as a stabilizer to prevent nanoparticle agglomeration. A solution of ascorbic acid was added to this mixture, which led to the reduction of Cu2+ ions to metallic copper. To create the alkaline environment necessary to activate the reduction process, a sodium hydroxide (NaOH) solution was introduced into the system. The reaction was carried out at a temperature of approximately 50 °C with constant stirring for 40-50 minutes until a stable colloid formed. A change in the reaction was indicated by a color change of the solution: the appearance of an intense dark reddish hue signaled the formation of copper nanoparticles.
Synthesis with Glucose:
Under similar conditions, a second series of reactions was conducted, using glucose as the stabilizer.
Before starting the second phase of the experimental research, a bleached cotton fabric was washed in distilled water to remove potential contaminants and impurities. After drying the fabric at room temperature, the samples were kept in a desiccator to stabilize their moisture content, which allowed for a more accurate mass measurement on an analytical balance.
Cotton fabric samples, each measuring 200 x 200 mm, were treated with a solution containing copper nanoparticles synthesized using the two different formulations:
-
• Sample No. 1 was treated with the gelatin based formulation;
-
• Sample No. 2 was treated with the glucose -based formulation.
The fabrics were impregnated using a laboratory two-roll padding machine with a pressing level of 90%. This was followed by drying at 110 °C, and then heat treatment at 120 °C on pin frames in a thermostated drying oven. After this, the samples were additionally rinsed with distilled water to remove any unfixed residues of the formulation and dried at room temperature.
The antimicrobial properties of the cotton fabric were tested using a laboratory method for resistance to microbial degradation.
Research and discussion
In the course of this study, two methods for the synthesis of copper nanoparticles based on «green chemistry» principles were developed and comparatively evaluated. Gelatin and glucose were chosen as stabilizers because both substances have high biocompatibility and contribute to the formation of stable colloidal systems.
The synthesis of copper nanoparticles was carried out by reducing an aqueous solution of copper sulfate. Ascorbic acid was used as the reducing agent, and gelatin as the stabilizer. To determine the optimal concentrations of the starting components, a series of experiments was conducted (Table 1).
Table 1. Concentrations of Starting Components
|
Sample number |
Concentration, g/mol |
||
|
CuSO 4 5 H 2 O |
C 6 H 8 O 6 |
Gelatin |
|
|
1 |
0,006 |
0,05 |
0,01 |
|
2 |
0,0125 |
0,1 |
0,01 |
|
3 |
0,0175 |
0,4 |
0,01 |

a)
Figure 1. Photographs of copper nanoparticles from Sample 1
Analysis of the obtained micrographs using scanning electron microscopy (SEM) showed that the morphology and size of the nanoparticles are significantly dependent on the type of stabilizer. When gelatin was used, the formation of particles with sizes ranging from 30 to 74 nm was observed, characterized by a more uniform shape and distribution.
In this synthesis, glucose acts as a stabilizer,

b)
(a), 1 (b) in the presence of a gelatin stabilizer
ensuring a uniform distribution of particles and preventing excessive clumping in the initial stages. However, despite the stabilizing effect, some of the nanoparticles tend to form loose aggregates. Such clusters do not completely disrupt the nanoscale nature but increase the average size of the observed particles. To determine the optimal concentrations of the starting components, a series of experiments was conducted (Table 2).
Table 2. Concentrations of initial components
|
Sample number |
Concentration, g/mol |
||
|
CuSO 4 5 H 2 O |
C 6 H 8 O 6 |
Glucose |
|
|
1 |
0.006 |
0.03 |
0,005 |
|
2 |
0,0125 |
0.03 |
0,011 |
|
3 |
0,0175 |
0.03 |
0,016 |
а) b)
Figure 2. Photographs of copper nanoparticles from Sample 1 (a) and Sample 2 (b) in the presence of a glucose stabilizer
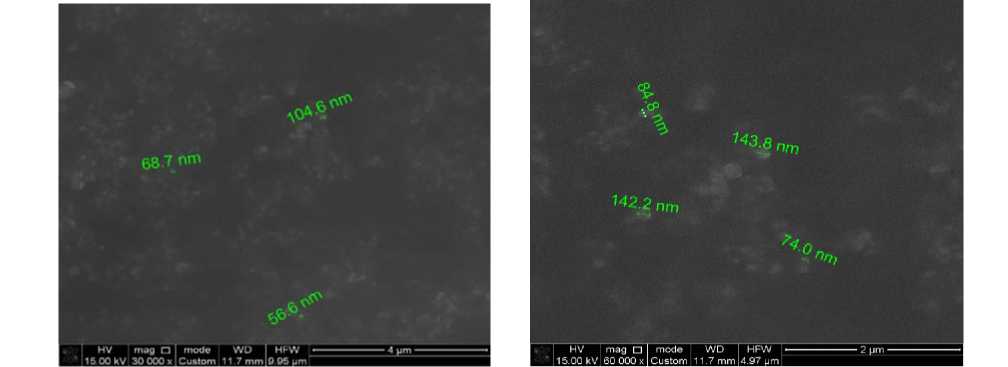
The results from the scanning electron microscopy (SEM) micrographs showed that when glucose was used as a stabilizer, the nanoparticle sizes varied in the range of 56 to 143 nm. This indicates a lower degree of stabilization and possible particle aggregation.
b)

а)
Figure 3. Micrographs of copper nanoparticles obtained by scanning electron microscopy (SEM) at various magnifications in the presence of a gelatin stabilizer
As a result of the scanning electron microscopy (SEM) micrographs obtained, it was shown that when glucose was used as a stabilizer, the nanoparticle sizes varied in the range of 56 to 143 nm. This indicates a lower degree of stabilization and possible particle aggregation.
To prepare the biocidal composition, tetraethoxysilane (TEOS) was used as a structureforming matrix, with ethyl alcohol as the solvent in a water-to-alcohol ratio of 4:1. Then, copper(II) sulfate pentahydrate and ascorbic acid as a reducing agent were added. Gelatin was used as the stabilizer in the first formulation, and glucose in the second. The preparation time was 40–50 minutes at a temperature of 50 °C.
Testing conducted at the Research Laboratory for the Assessment of Food Product Quality and Safety at Almaty Technological University JSC established that the samples demonstrated a zone of inhibition of E. coli growth in the range of 0.2–0.4 mm (when gelatin was used as a stabilizer) and 0.2–0.3 mm (when glucose was used), which indicates pronounced antimicrobial activity. The introduction of TEOS into the impregnation solution contributed to the formation of a silicate matrix on the fiber surface, ensuring a stronger fixation and stabilization of the copper nanoparticles, thereby increasing the effectiveness of the antimicrobial action..
Table 3. Preparation of solution based on stabilisers (gelatin/glucose) and tetraethylorthosilicate
|
Name of reagents |
Sample No.1 (g/mol) |
Sample No.2 (g/mol) |
Sample No.3 (g/mol) |
|
temperature to– 50 о С |
|||
|
CuSO4 5 H2O |
0,006 |
0,0125 |
0,0175 |
|
С 6 Н 8 О 6 |
0,03 |
0,03 |
0,03 |
|
Gelatin |
0,01 |
0,01 |
0,01 |
|
Glucose |
0,005 |
0,011 |
0,016 |
|
Tetraethylorthosilicate (TEOS) |
0,03 |
0,03 |
0,03 |

а) stabiliser gelatine
b) stabiliser glucose
Figure 4. Treated samples with the addition of tetraethyl orthosilicate and stabilizers: (a) gelatin, (b) glucose
Thus, the addition of TEOS to the impregnation solution allowed for partial antibacterial activity against E. coli . This confirms the feasibility of further research into the effect of TEOS on nanoparticle stabilization and the enhancement of the antimicrobial effect.
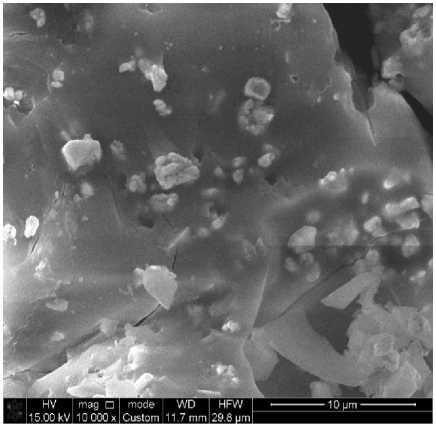
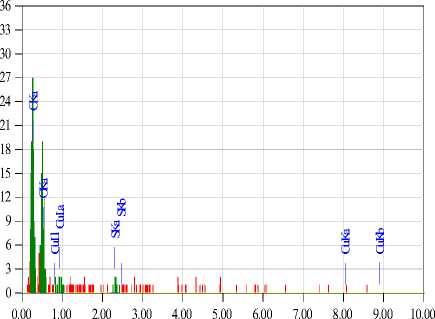
Scanning electron microscopy (JET 6300) revealed a change in the morphological surface of the treated samples compared to the untreated ones (Fig. 5). The presence of a silicon oxide matrix is observed on the surface of the treated fabrics. The proposed method ensures the fixation of copper sulfate nanoparticles on the surface of the cotton fabric. Elemental analysis confirmed the presence of substances on the fabric surface. It can also be noted from the results of the elemental analysis that in the antimicrobial cotton fiber of the first sample, where gelatin is the stabilizer (Fig. 6), the structure contains 1.9% Si and 0.93% Cu. In the case of the second sample (Fig. 7), where glucose is the stabilizer, the composition is 1.73% Si and 0.93% Cu.

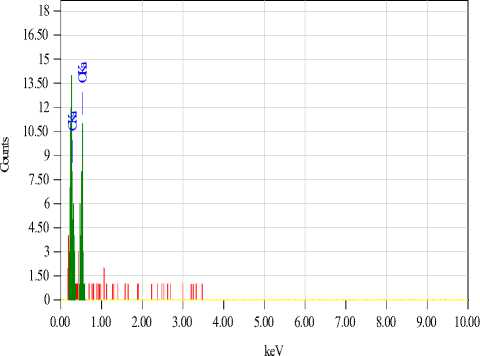
Fig. 5 - Electron scanning microscopy image of an untreated sample

keV
a)

Fig. 6. Electron-scanning microscopy image of treated samples (a- stabiliser glucose; b) stabiliser gelatin).
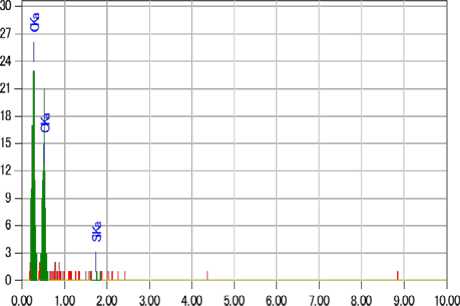
keV
b)
Based on what has been said, it can be concluded that the proposed method of antimicrobial treatment based on copper sulfate with the addition of tetraethoxysilane provides pronounced antimicrobial activity to textile materials.
The difference in morphological characteristics directly impacted how the solutions interacted with the cotton fabric. Treating the textile with the solution containing gelatin-stabilized particles resulted in a more uniform distribution on the fabric surface, which could potentially increase the modified material's resistance to biological influences. Samples treated with the glucose-based formulation showed a less homogeneous distribution, which is also confirmed by SEM analysis.
Thus, the choice of stabilizer plays a key role not only in the nanoparticle synthesis process but also in the effectiveness of the subsequent modification of textile materials.
Conclusion
An environmentally friendly method for synthesizing copper nanoparticles using ascorbic acid as a reducing agent and gelatin and glucose as stabilizers was successfully developed through experimental research. It was established that gelatin promoted the formation of smaller, more uniform nanoparticles (30– 74 nm), while glucose resulted in significantly larger particles (56–143 nm).
Treated fabrics using copper sulfate with ascorbic acid and a stabilizer (gelatin or glucose), along with the addition of tetraethoxysilane (TEOS), formed a silicate matrix. This matrix led to better fixation and stabilization of the copper nanoparticles on the fibers and achieved a positive antimicrobial effect. The zones of inhibition for E. coli growth were 0.2–0.4 mm for samples with gelatin and 0.2–0.3 mm for samples with glucose.
Scanning electron microscopy analysis confirmed the effectiveness of the proposed method for modifying cotton fabrics. The samples treated with the silicon oxide matrix ensured the fixation of copper sulfate nanoparticles on the fiber surface, which contributed to the formation of antimicrobial properties. It was determined that the sample with the gelatin stabilizer had a silicon content of 1.9% and a copper content of 0.93%, while the sample with the glucose stabilizer had 1.73% silicon and 0.93% copper. This shows stable fixation of the functional components regardless of the stabilizer type.
In conclusion, the developed TEOS-based compositions can be considered promising for creating functional textile materials with antimicrobial and protective properties. The data presented can be used to optimize synthesis parameters and improve the effectiveness of nanostructured coatings in the textile industry.
Funding
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP26195961, «Development of technology for obtaining new textile materials with special properties»).


